Types of Receptors
Cellular receptors are protein macromolecules that are essential for cell signaling. Like every lock has a specific key, every receptor molecule only recognizes and responds to a particular signaling molecule (ligand). This ligand binding triggers a change in the receptor, leading to a series of downstream signaling actions.
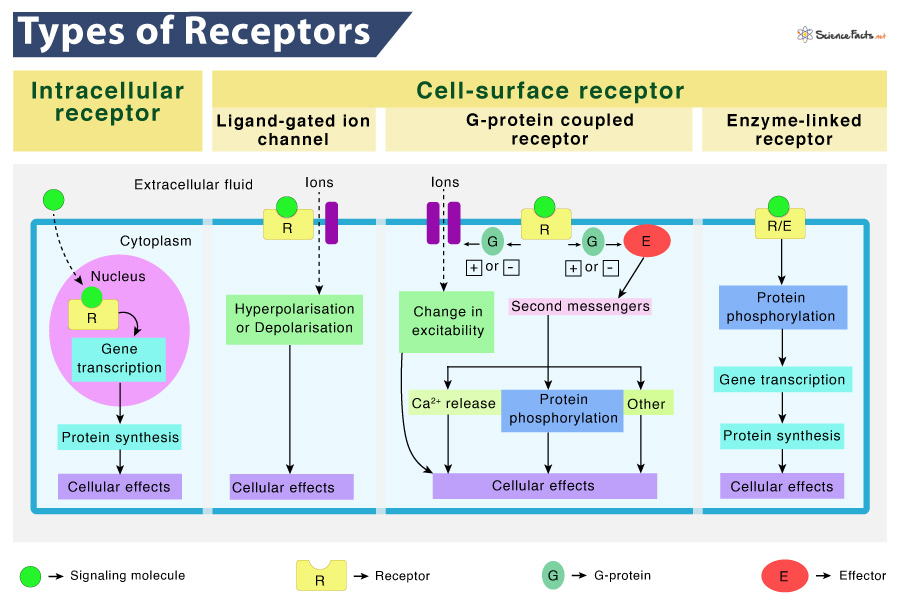
Receptors are broadly categorized into two types based on their location. They are intracellular receptors (found inside the cell) and cell-surface receptors (found in the plasma membrane).
1) Intracellular (Internal) Receptors
As mentioned, intracellular or internal receptors are found inside the cell, mainly in the cytoplasm or nucleus. In most cases, their ligands are small, hydrophobic, lipid-soluble molecules. They must be able to travel through the cell membrane to reach their respective receptors.
For example, the primary receptors for hydrophobic steroid hormones, such as the sex hormones estradiol (an estrogen) and testosterone, are intracellular.
These receptors comprise three core domains: a ligand-binding domain, DNA binding domain, and an amino terminus (which interacts with the gene transcription machinery).
Once the ligands reach the cell interior, they bind to their specific intracellular receptor to form a ligand-receptor complex. Then it triggers a conformational change that exposes a DNA-binding site on the protein. The ligand-receptor complex moves into the nucleus, then bind to specific regulatory regions of the chromosomal DNA and promote transcription initiation, i.e., producing mRNA from DNA.
Thus, the internal receptors can directly influence gene expression without passing the signal to other receptors or messengers.
2) Cell-surface Receptors
Cell-surface receptors, alternatively known as transmembrane receptors, are membrane-anchored or integral proteins found attached to the external or outer surface of the cell membrane. Most of the signaling in multicellular organisms is conducted by these receptors. The ligands are large, hydrophilic, and cannot pass through the cell membrane, unlike the previous type. So, they bind to these receptors to ferry across the plasma membrane.
These receptors are also called cell-specific proteins or markers as they are specific to individual cell types. Cell-surface receptor spans the cell membrane and performs signal transduction by converting an extracellular signal into an intracellular one. Here, the ligands do not have to enter the affecting cell.
A typical cell-surface receptor contains three domains. They include an extracellular ligand-binding domain, a hydrophobic membrane-spanning domain, and a signal transmitting intracellular domain. The size and structure of these regions can vary depending on the type of receptor. Also, the hydrophobic region may consist of multiple stretches of amino acids that traverse through the membrane.
Cell-surface receptors are of the following three types:
a) Ligand-gated Ion Channels
Ligand-gated ion channels are a subtype of ion channels that can open in response to the binding of their respective ligand. When a ligand adheres to the extracellular region of the channel, a conformational change occurs in the protein’s structure, allowing ions such as sodium, calcium, magnesium, and hydrogen to pass through.
This type of cell-surface receptor contains an extensive membrane-spanning region with a hydrophilic channel through its middle. The ions cross the membrane through this channel without touching the hydrophobic core of the phospholipid bilayer.
In order to do so, many of the amino acids in the membrane-spanning region are hydrophobic. Conversely, the amino acids lining the inside of the channel are hydrophilic, allowing water or ions to pass.
Neurons, or nerve cells, have ligand-gated channels that are bound by neurotransmitters.
b) G protein-Coupled Receptors
G protein-coupled receptors (GPCRs) belong to an extensive family of cell surface receptors, having the same structure and signaling method. All the family members possess seven transmembrane domains and transmit signals inside the cell through G protein. However, each receptor has its specific extracellular domain and G-protein-binding site.
GPCRs bind to a variety of ligands. Among them, an interesting one is odorant (scent) receptors.
Cell signaling via GPCRs occurs as a cyclic series. Before binding a ligand, the inactive G-protein can adhere to a newly-exposed site on the receptor specific for its binding. Once the G-protein binds to the receptor, a conformational change occurs, activating the G-protein. After activation, it releases GDP and picks up GTP. In the next step, the G-protein splits into α and β subunits. One or both of these G-protein fragments may be able to activate other proteins as a result. Later, the GTP on the active α subunit of the G-protein is hydrolyzed to GDP, and the β subunit is deactivated. Lastly, the subunits associate again to form the inactive G-protein, and the cycle starts over.
c) Enzyme-Linked Receptors
Enzyme-linked receptors are a class of cell-surface receptors whose intracellular domains are associated with an enzyme. For some, the intracellular domain of the receptor is itself an enzyme, catalyzing a reaction. Other types have an intracellular domain that directly interacts with an enzyme.
These receptors generally possess large extracellular and intracellular domains. However, their membrane-spanning zone consists of a single alpha-helical peptide strand.
When a ligand binds to its extracellular domain, a signal is transferred through the membrane. The signal then activates the enzyme and sets off a series of events inside the cell.
Receptor tyrosine kinases (RTKs) are a class of enzyme-linked receptors found in humans and many other species. This receptor transfers phosphate groups to tyrosine molecules. The signaling molecules bind to the extracellular domain of two nearby receptors, which dimerize afterward. Then, phosphates are added to tyrosine residues on the intracellular domain of the receptors, which then convey the signal to the next cytoplasmic messenger.
-
References
Article was last reviewed on Thursday, February 2, 2023