Rough Endoplasmic Reticulum
What is Rough Endoplasmic Reticulum
Rough endoplasmic reticulum (RER), also known as granular ER, is a type of endoplasmic reticulum, a membrane-bound cellular organelle found in all eukaryotic cells, including both plant and animal cells. The main function of RER is synthesis, folding, modification, and transport of proteins to different target organelles within or outside the cell.
Where is it Located
The RER is continuous with the nuclear membrane, which envelopes the cell nucleus. Being a part of the endomembrane system, it also lies near the Golgi apparatus. Moreover, the rough endoplasmic reticulum arises near the nucleus and stretches across the cytoplasm, up to the Golgi body.
Structure
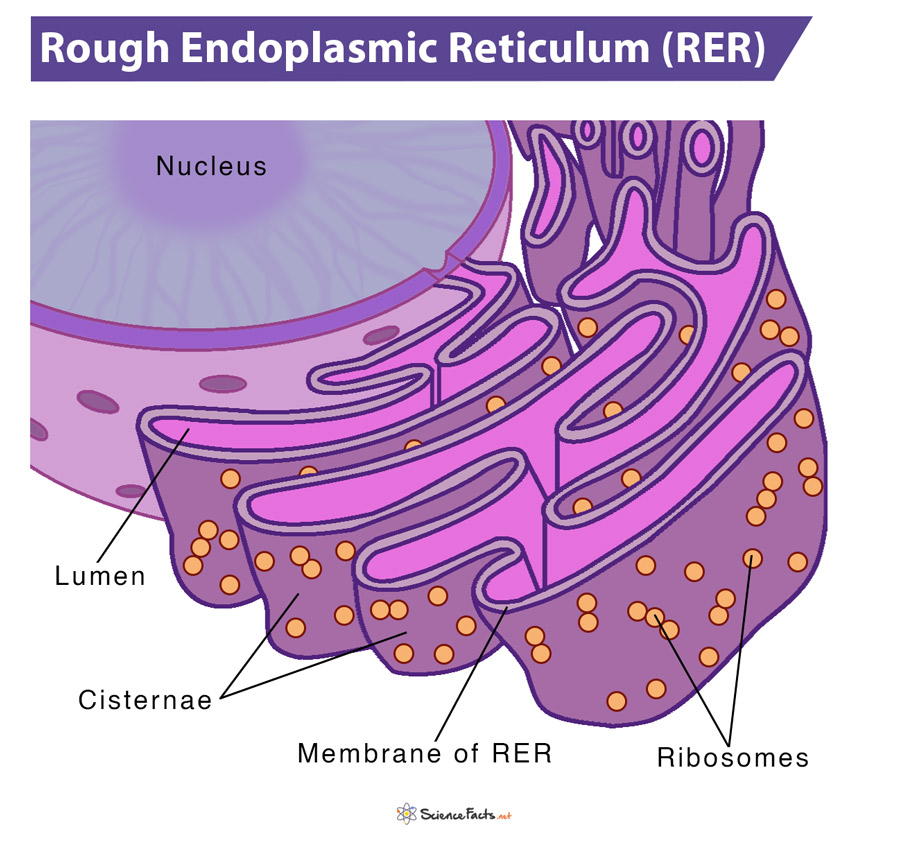
The RER is primarily made of an array of membrane-enclosed, interconnected flattened sacs, called cisternae. The area enclosed by the membrane of RER is referred to as the lumen.
Their unit membranes have ribosomes studded on their outer surface, which make them the ‘rough’ ER. The ribosomes remain attached with the help of a group of proteins called ribophorins. In addition to ribosomes, these membranes feature another protein complex called the translocon, which is necessary for protein translation within the lumen of RER. So, overall the RER looks like a stack of flattened sacs studded with bumpy ribosomes.
The main structural framework of rough ER is dependent on cytoskeletal elements, especially microtubules. If the microtubule structure gets disrupted, the ER network collapses and reforms only after reestablishing the cytoskeleton.
Functions
The primary function of the rough endoplasmic reticulum is the synthesis, folding, modification, and transport of proteins to their targeted destinations.
1. Protein Synthesis: The process of protein synthesis occurs in two main steps: transcription and translation.
Transcription:
i) In this step, DNA is used as a template to produce messenger RNA (mRNA).
ii) The mRNA thus produced, leaves the nucleus and approaches a ribosome in the cytoplasm, where translation occurs.
Translation:
i) Here, the genetic code in mRNA is read and used to make a polypeptide.
ii) If the polypeptide contains a signal sequence at its amino-terminal end, it binds to a signal recognition particle (SRP), which carries the ribosome to the RER membrane.
ii) Upon reaching the RER, the nascent polypeptide is threaded into the organelle through transmembrane channels called translocons.
iii) Once bound, the SRP dissociates, and protein translation continues. If the newly formed protein is a transmembrane protein, it remains embedded in the RER membrane. Otherwise, if it is a water-soluble protein, it gets transmitted into the RER lumen via a translocon channel.
iv) New amino acids are added to the growing polypeptide chain as the ribosome remains attached to the RER membrane, and the nascent protein continues to be inserted into the lumen. This process is termed as co-translational import into the RER.
2. Protein Folding and Quality Control: Next, the short stretches of polypeptide chains must be folded to their final 3-D structure by undergoing slight modifications.
Protein folding:
After synthesis, the signal sequences of polypeptide chains are cleaved, and they go through a process known as glycosylation. It increases the solubility of the peptide chains and protects them until molecular chaperones bind to them and facilitate their folding. Some examples of molecular chaperones are binding immunoglobulin protein (BiP), Calnexin (CNX), and Calreticulin (CRT). Glycosylation of the nascent polypeptide occurs under the influence of enzyme oligosaccharyltransferases, a part of the translocon complex of the RER membrane. Here, an oligosaccharide is added to the chain, producing a glycoprotein.
Quality control:
Despite these mechanisms, some polypeptide chains cannot be folded correctly either due to translation errors or genetic mutations, which leads to the production of defective proteins. The quality control systems within the RER proofread each newly synthesized protein and try to fix the problem. When the polypeptide has not folded into its correct form, molecular chaperones rebind to the polypeptide and again attempt to fold the protein into its correct shape. After repeated failed attempts, misfolded proteins are exported to the cytosol.
3. Protein Sorting: After the proteins are synthesized and folded, they go to the edges of the RER. Then, secretory vesicles start to form from the edges of the rough ER, mediated via vesicular coat protein complex II (COPII). These vesicles carry cargo towards the Golgi network for further processing. Proteins that need to stay within the ER are moved back through retrograde transport from the Golgi using vesicles formed by coat protein complex I (COPI).
Note: An analogy that can be made to describe the role of the rough endoplasmic reticulum is that of a factory assembly line. In a factory, the workers gather the produced goods, assemble them and deliver them to their designated places. Likewise, the RER also acts as a factory worker, by gathering the proteins produced by ribosomes, modifying them, and transporting them to their targeted destinations.
-
References
Article was last reviewed on Friday, May 27, 2022