Proton
What is a Proton
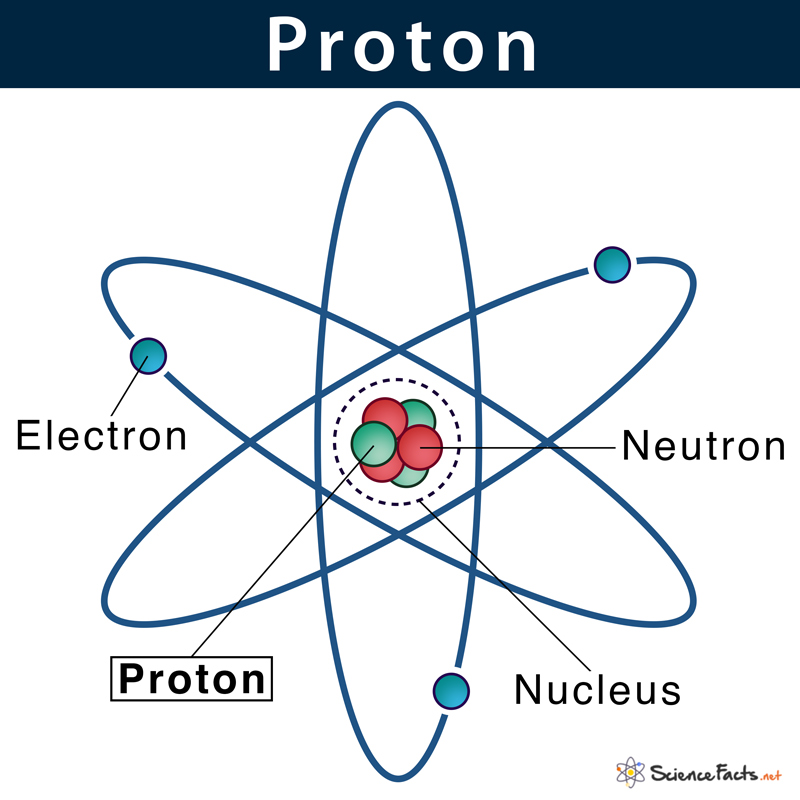
A proton is one of the three main subatomic particles that make up an atom. The other two being neutron and electron. The common symbol for a proton is p or p+. The nucleus of a hydrogen atom or H+ ion is an example of a proton.
The word ‘proton’ came from the Greek word meaning ‘first’. Ernest Rutherford used the term for the first time in 1920 to describe the nucleus of hydrogen. William Prout put discovered the existence of protons in 1815.
Who Discovered Proton
The presence of a positively charged particle in an atom was first observed in 1886 by Eugen Goldstein. It was discovered based on the concept that atoms are electrically neutral. Later in 1917, Ernest Rutherford discovered proton in his famous gold foil experiment.
Where are they Located?
Protons are found inside the atom’s nucleus, a tiny dense region at the center of the atom.
What are Protons Made of?
They are made of fundamental particles called quarks and gluons. A proton contains three quarks (two up quarks and one down quark) and three streams of gluons. Although protons were discovered almost a hundred years ago, quarks were discovered by Murray Gell-Mann and George Zweig in 1964, while Gluonswere discovered by John Ellis and his fellow workers in 1979.
Characteristics
1. Size: Not being a fundamental particle, it holds a measurable size. The root-mean-square charge radius of a proton is 0.84×10−15 to 0.87×10−15 m.
2. Charge: Has a positive electrical charge of +1, which is equal and opposite in magnitude to the charge of an electron. Mathematically, charge on a proton is given as + 1.602 × 10-19 coulombs.
3. Mass: It has a mass equal to that of a hydrogen atom. Mathematically, it has a mass of one atomic mass unit (amu), which is about 1.672 x 10-24 g. This weight is roughly 1,836 times the mass of an electron. Together with neutrons, they make up virtually all of the mass of an atom.
4. Number in Atoms: Every nucleus of a given element has the same number of protons. The number of protons in an atom’s nucleus is equal to the number of negatively charged electron revolving around the nucleus. For example, the number of protons in the element Osmium is 76.
5. Movement: They travel in a straight line and cast a shadow of the object placed in their path.
6. Other: All protons are identical, meaning a proton of hydrogen is similar to Helium or any other elements. However, its number differs in different elements.
-
References
Article was last reviewed on Thursday, February 2, 2023