Osmoregulation
Osmoregulation is the process of maintaining proper water and salt balance in the body of an organism. It regulates the osmotic pressure of the body fluids with the help of osmoreceptors.
In unicellular organisms such as bacteria, the process is relatively simple as it is done with the help of cell membranes through diffusion. In contrast, the body of a multicellular organism does not exist in isolation. They constantly interact and exchange water and nutrients with the environment and excrete sweat, urine, and faces.
Osmoregulation is thus vital for the normal functioning of all single-celled or multi-celled organisms on Earth.
Types of Osmoregulation
Organisms can perform osmoregulation in two ways. Accordingly, the two categories of organisms are:
1. Osmoconformers
These organisms maintain the same osmolarity of their body fluids as the surrounding environment. It requires the intake and outflow of water and salts to be equal over an extended period, which occurs using an active or passive transport mechanism.
Most marine invertebrates are osmoconformers, starfish, jellyfish, sharks, crabs, earthworms, and lobsters.
2. Osmoregulators
Such organisms regulate their internal osmotic pressure irrespective of the surrounding environment. They maintain a constant balance between the outside and inside environment.
Osmoregulators are most common in animal environments, like most freshwater fish, salmon, eel, and many vertebrates, including humans.
Osmoregulators vs. Osmoconformers
Thus, the main difference is that osmoregulators try to match the osmolarity of their body to the outside environment. In contrast, osmoconformers maintain a constant internal body osmolarity, the same as the surrounding environment.
Why is Osmoregulation Important
At the organism level, osmoregulation helps to maintain the concentration of salt, electrolytes, and water inside the body, which are critical for carrying out their metabolic activities. They also help remove toxic waste products from the body. Without osmoregulation, toxic substances will accumulate, leading to the organism’s death.
At the cellular level, the osmolarity of the extracellular fluid impacts the exchange of water with the cell interior. Thus, according to the relative concentration of fluid and salts and the permeability of cell membrane (tonicity), there are three possible situations:
- In isotonic solution (same solute concentration in and out of the cell), there is no net water flow.
- In hypertonic solution (higher concentration of solute than inside the cell), fluids move out of the cell, causing the cell to shrink.
- In a hypotonic environment (lower concentration of solute than inside the cell), water moves inside, causing the cell to swell.
We will now see how osmoregulation works in different organisms, including fishes, bacteria, protozoa, and humans, and how it helps them to survive.
Osmoregulation in Fishes
The process of osmoregulation is most widely studied in marine organisms, especially fishes. Most freshwater and marine fishes exhibit osmoregulation to survive.
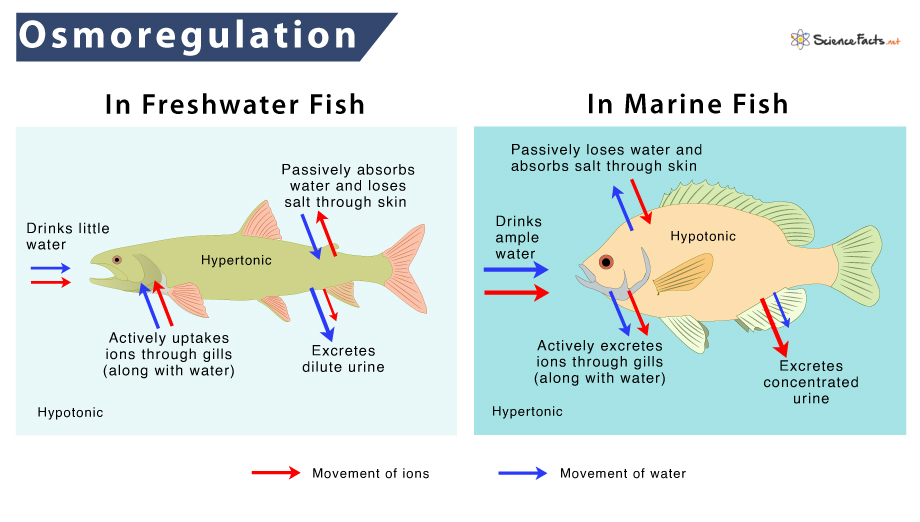
Freshwater Fish
The inside of the body of these fishes has a higher concentration of salt than the external environment. The fish is thus in a hypotonic environment. Subsequently, there is a tendency to lose salt and absorb water.
To compensate for the loss, freshwater fish have very efficient kidneys that excrete water quickly. They also reabsorb salt from their urine and actively take salt from their environment using special cells in the gills.
Marine Fish
In such fishes, the situation is exactly opposite to that in freshwater fishes. They lose water and gain salt, as their bodies have more salt and less water than their environment.
Marine fish take in vast amounts of water and pass less urine to tackle this situation. Special cells in the gills eliminate salt but do not absorb it from the water they take.
Osmoregulation in Humans
Humans must maintain a stable salt and water balance inside the body to maintain a stable internal environment (homeostasis). They do so using excretory organs, kidneys, and skin.
Through Kidneys
When the water level in the body rises, it releases dilute or hypotonic urine. In contrast, when the water level drops, it produces a low amount of concentrated or hypertonic urine. The kidneys also reabsorb salt, water, and glucose from the filtrate to compensate for the electrolyte loss. The hormones that help in this process are Aldosterone, angiotensin II, and antidiuretic hormones (ADH).
Through Skins
Skins also maintain the internal salt and water balance through sweating or perspiration, which allows the release of fluids through the spores of the skin.
Osmoregulation in Plants
Similar to humans, plants also maintain osmolarity by releasing water through minute pores called stomata. Plants growing in dry areas have thick and fleshy cuticles to prevent water loss, storing water in the vacuoles. In contrast, aquatic plants release excess water and later compensate for the loss by absorbing it from their environment.
Osmoregulation in Microorganisms
Bacteria respond to osmotic stress by rapidly taking in electrolytes or small organic solutes via membrane transport proteins called transporters. The function of transporters increases when there is an increase in osmolarity. They also activate stress-response genes to cope with the situation.
Protozoa use contractile vacuoles to collect excretory wastes, such as ammonia, from the intracellular fluid by diffusion and active transport. As osmotic action pushes water from the environment into the cytoplasm, the vacuole simultaneously moves to the surface and pumps the contents into the environment.
Thus, maintaining a proper osmotic balance is essential for proper cell functioning, thus keeping the cell or organism healthy.
The concept of osmoregulation also has some clinical significance, such as maintaining body fluids in patients and proper functioning of the nephron.
FAQs
Ans. Excretion is the process by which metabolic wastes are eliminated from an organism. In contrast, osmoregulation is the process of regulating the osmotic potential of the cell within an organism.
-
References
Article was last reviewed on Thursday, February 2, 2023