Miller-Urey Experiment
The Miller-Urey Experiment was a landmark experiment to investigate the chemical conditions that might have led to the origin of life on Earth. The scientist Stanley Miller, under the supervision of the Nobel laureate scientist Harold Urey conducted it in 1952 at the University of Chicago. They tried to recreate the conditions that could have existed in the first billion years of the Earth’s existence (also known as the Early Earth) to check the said chemical transformations.
Miller-Urey Experiment And The Primordial Soup Theory
The experiment tested the primordial or primeval soup theory developed independently by the Soviet biologist A.I. Oparin and English scientist J.B.S. Haldane in 1924 and 1929 respectively. The theory propounds the idea that the complex chemical components of life on Earth originated from simple molecules occurring naturally in the reducing atmosphere of the Early Earth, sans oxygen. Lightning and rain energized the said atmosphere to create simple organic compounds that formed an organic “soup”. The so-called soup underwent further changes giving rise to more complex organic polymers and finally life.
The Miller-Urey Experiment In Support Of Abiogenesis
From what was explained in the previous paragraph, it can undoubtedly be considered as a classic experiment to demonstrate abiogenesis. For those who are not conversant with the term, abiogenesis is the process responsible for the development of living beings from non-living or abiotic matter. It is thought to have taken place on the Earth about 3.8 to 4 billion years ago.
Miller-Urey Experiment Apparatus and Procedure
The groundbreaking experiment used a sterile glass flask of 5 liters attached with a pair of electrodes, to hold water (H2O), methane (CH4), ammonia (NH3) and hydrogen (H2), the major components of primitive Earth. This was connected to another glass flask of 500 ml capacity half filled with water. On heating it, the water vaporized to fill the larger container with water vapor. The electrodes induced continuous electrical sparks in the gas mixture to simulate lightning. When the gas was cooled, the condensed water made its way into a U-shaped trap at the base of the apparatus.
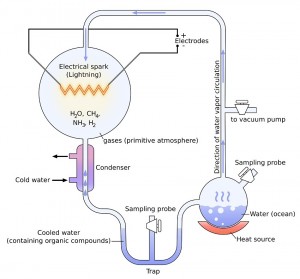
After electrical sparking had continued for a day, the solution in the trap turned pink in color. At the end of a week, the boiling flask was removed, and mercuric chloride added to prevent microbial contamination. After stopping the chemical reaction, the scientist duo examined the cooled water collected to find that 10-15% of the carbon present in the system was in the form of organic compounds. 2% of carbon went into the formation of various amino acids, including 13 of the 22 amino acids essential to make proteins in living cells, glycine being the most abundant.
Though the result was the production of only simple organic molecules and not a complete living biochemical system, still the simple prebiotic experiment could, to a considerable extent, prove the primordial soup hypothesis.
Miller-Urey Experiment Animation
Chemistry Of The Miller And Urey Experiment
The components of the mixture can react among themselves to produce formaldehyde (CH2O), hydrogen cyanide (HCN) and other intermediate compounds.
CO2 → CO + [O] (atomic oxygen)
CH4 + 2[O] → CH2O + H2O
CO + NH3 → HCN + H2O
CH4 + NH3 → HCN + 3H2
The ammonia, formaldehyde and HCN so produced react by a process known as Strecker synthesis to form biomolecules including amino acids.
CH2O + HCN + NH3 → NH2-CH2-CN + H2O
NH2-CH2-CN + 2H2O → NH3 + NH2-CH2-COOH (glycine)
In addition to the above, formaldehyde and water can react by Butlerov’s reaction to produce a variety of sugars like ribose, etc.
Though later studies have indicated that the reducing atmosphere as replicated by Miller and Urey could not have prevailed on primitive Earth, still, the experiment remains to be a milestone in synthesizing the building blocks of life under abiotic conditions and not from living beings themselves.
-
References
Article was last reviewed on Thursday, February 2, 2023








This experiment is currently seen as not sufficient to support abiogenesis.
See Stephen C. Meyer, James Tour.
These experiments did not produce modern biologic Amino acids; such as Proline, Glutamate or Lysine. They only produced rudimentary parts: Origin of life science has failed to show us ALL the steps that take us from these simple precursory molecules to just the individual chain parts of the larger peptide molecules!! Your analysis is ponderously incomplete <<