Isotonic Solution
What is an Isotonic Solution
If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’).
It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. Here, the solute concentration, i.e., osmolarity (solutes per liter), remains equal on both sides of the cell membrane.
Some of the most commonly used isotonic solutions are normal saline with 0.9% NaCl and lactated ringers.
What It Does to a Cell
A cell always tries to achieve an equilibrium state if there is a difference in concentration between its internal and external environment. It does so by a continuous influx or efflux of water and other nutrients. This activity ensures a constant concentration or osmotic pressure in and out of the cell. As a result, the size and shape of a cell remain intact, maintaining its stable structure.
Any living being, including humans, possess some regulatory mechanisms to achieve and maintain this homeostatic condition which helps them to carry out all the body functions with ease.
What Happens to a Cell When Placed in an Isotonic Solution
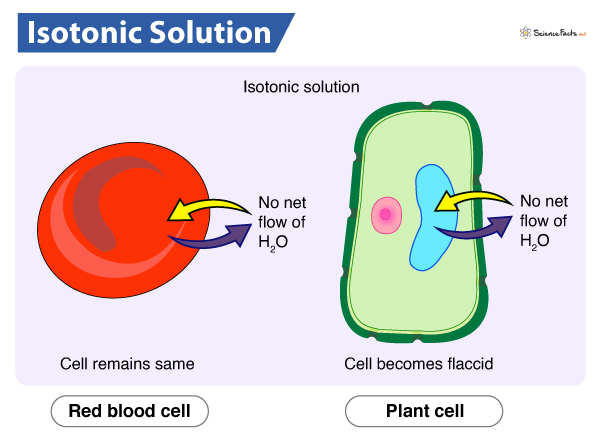
When we expose a cell to an isotonic solution, there is no exchange of water in and out of the cell and vice versa. Thus, the cell size remains constant.
As mentioned, an isotonic solution has the same solute concentration and osmotic pressure both inside and outside of the cell. Generally, solutes and water move following their concentration gradient (from higher to lower concentration), but this difference does not exist in case of isotonic solutions. Consequently, there is no net flow of water, i.e., osmosis does not occur.
Examples of Isotonic Solution
In Animals
Animal cells tend to favor an isotonic environment. When placed in a hypotonic solution, the cells swell up due to an excessive influx of water and may even burst as they lack a cell wall. On the contrary, water moves out of the cells if kept in a hypertonic solution, dehydrating them. As a result, they lose their structure, appearing wrinkled. So, animals have regulatory mechanisms to control the osmolarity and maintain an isotonic environment in both the internal and external environment of the cell (osmoregulation).
For instance, freshwater fishes, such as catfish and gourami produce very dilute urine to prevent the loss of salts from their body. Thus, it helps them to keep their salt and water balance in check.
In Blood Cells
Blood plasma is generally isotonic to the cytoplasmic concentration of blood cells. These cells tend to maintain this isotonic environment both internally and externally, as osmotic pressure greatly influences blood pressure. Also, they aim to maintain isotonicity to prevent the swelling or shrinking of the blood cells.
In Plants
In plants, the transport of various essential solutes and water occurs across the cell membrane following their concentration gradient, i.e., from higher to lower concentration. In case of an isotonic solution, no difference in concentration exists between the internal and external environment of the cell. As a result, no exchange of nutrients and water take place. Plants do not receive their required nutrition, and lack of water makes them appear wilted. So, plants usually prefer a hypotonic environment over an isotonic one.
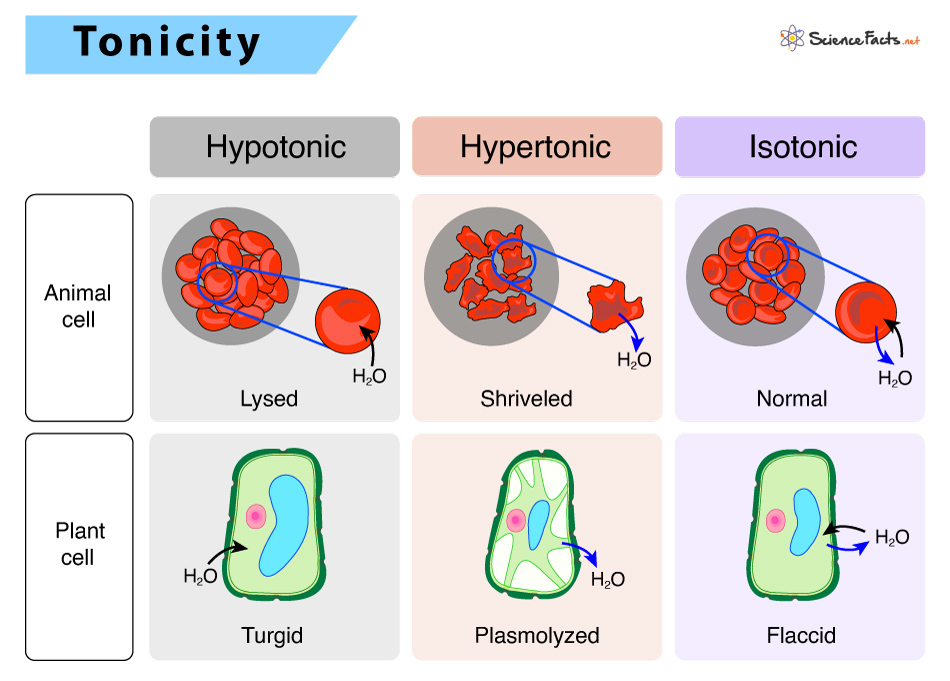
Difference between Isotonic, Hypotonic, and Hypertonic Solutions
Based on tonicity, i.e., relative concentrations of the solutions, solutions can be broadly classified into three types: Isotonic, hypotonic, and hypertonic.
The table below shows the comparison between these three types of solutions:
| Isotonic Solution | Hypotonic Solution | Hypertonic Solution |
|---|---|---|
| Have equal solute concentration in and out of the cell | Have lower solute concentration in the extracellular fluid (ECF) | Have higher solute concentration in the extracellular fluid (ECF) |
| Equal osmotic pressure exists on both sides of the cell membrane | Lower osmotic pressure in ECF | Greater osmotic pressure in ECF |
| No net flow of water | Water moves into the cell | Water moves out of the cell |
| No effect on cells | Cells swell | Cells shrink |
FAQs
Ans. A 0.9% NaCl solution is said to be isotonic to a red blood cell.
Ans. The extracellular fluid (ECF) contains equal solute concentration in an isotonic solution than the cell interior. Whereas, for hypertonic and hypotonic, the ECF has higher and lower solute concentrations, respectively.
Ans. Hypotonic solutions: Freshwater, tap water, normal saline (0.45% NaCl).
Isotonic solutions: Normal saline (0.9% NaCl), lactated ringers.
Hypertonic solutions: Seawater, sugar syrup, corn syrup.
-
References
Article was last reviewed on Thursday, December 23, 2021