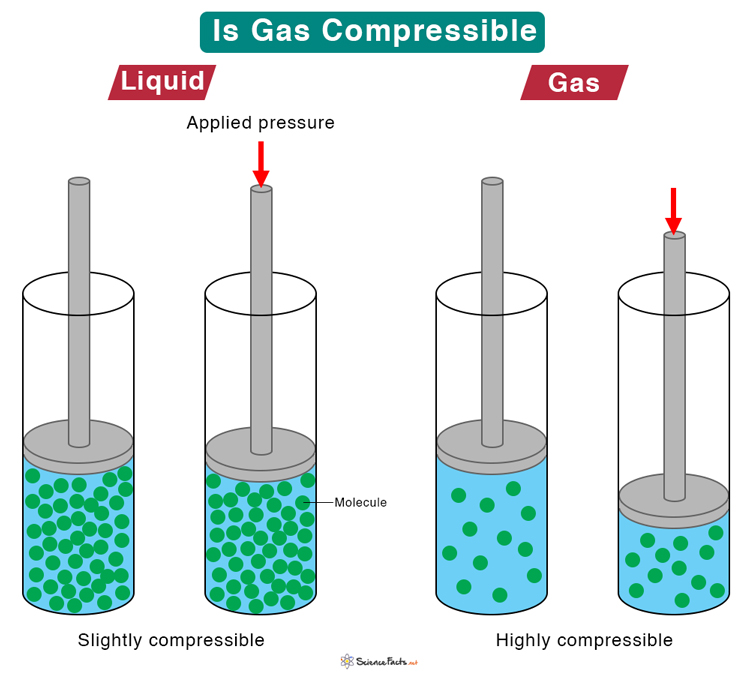
Is Gas Compressible?
Matter is composed of molecules that are either tightly or loosely packed. If the molecules are tightly packed, they cannot move freely, and matter cannot be compressed easily. For example, the molecules in solids and liquids are dense and do not move freely. Hence, solids and liquids are not easily compressible. On the other hand, if molecules are loosely packed, they can move freely, and matter can be compressed. Gas molecules move freely, and gas is easily compressible.
Why is a Gas Easier to Compress
According to the kinetic theory of gases, gas molecules are widely spread out at standard temperature and pressure. In other words, there is ample space between the molecules. The distance between the gas molecules is much more than their size. As a result, they can be brought closer through compression. The low density of gas makes it easier to compress than liquid.
What Happens to Gas Particles When a Gas is Compressed
A gas takes the shape and size of the container it is filled in. The gas particles move randomly inside the container. They collide with other particles and with the container walls. These collisions with the walls define the pressure of the gas. When a gas is compressed, its volume decreases, and pressure increases. The frequency of collision among the particles also increases. As a result, they generate heat and increase the temperature.
Where is Compressed Gas Used
Compressed gas has many applications. In a gas tank, gas is compressed and stored. For example, oxygen is compressed in a tank that is used to supply oxygen to hospital patients. A scuba diver carries an oxygen tank with him to breathe underwater. Propane gas is compressed into a liquid and stored in a cylinder. It is used to supply the fuel needed for cooking and welding.
-
References
Article was last reviewed on Friday, April 21, 2023