Electron
What are Electrons
Electrons are one of the three types of subatomic particles that make up an atom. The other two types are protons and neutrons. Unlike protons and neutrons, electrons are fundamental particles that cannot be divided further into smaller particles. The standard symbol for an electron is e or e-. Lightning during the rainy season is a natural source of electrons that powers various devices such as electric bulbs, television sets, motors, and mobile phones.
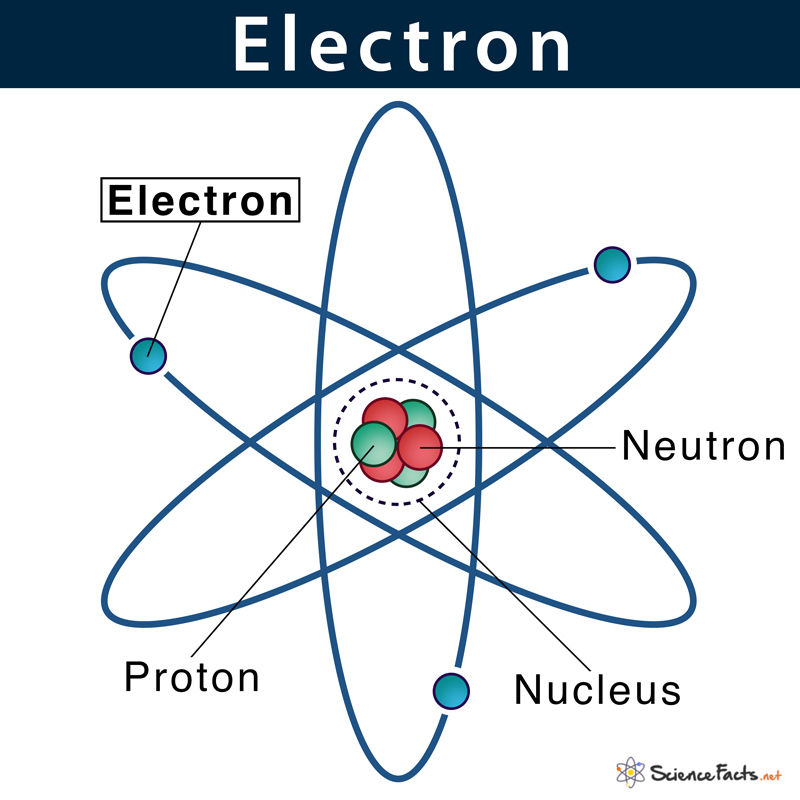
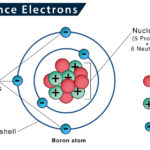
Given below is the structure of an atom, where electrons are moving around the nucleus. This pattern is similar to the way planets in the solar system revolve around the sun.
Who Discovered Electron
Richard Laming, G. Johnstone Stoney, and others first predicted the possibility of electrons in an atom. Later Stoney gave the term ‘electron’ in 1891. However, it was discovered by British physicist J.J. Thomson in the year 1897.
Where are they Found in an Atom
Electrons are located at fixed distances outside the atomic nucleus in pathways known as electron shells or orbitals. An atom’s electronic configuration gives the address of an electron in an atom.
Characteristics
1. Size: They are tiny in size, with an approximate radius of 2 × 10−10 cm.
2. Charge: They are negatively charged with a charge of -1, which is equal and opposite to the charge of a proton.
3. Mass: Has a mass of 9.1094 x 10-28 g, which is almost 1/2000 times the mass of proton or neutron. Thus contribute very less to the total mass of an atom.
4. Number in Atoms: All atoms have the same number of electrons as protons, thus helping the cell become electrically neutral.
Thus in a electrically neutral atom,
Number of electrons (e–) = Number of protons (p+)
For example, the nucleus of Osmium contains 76 electrons.
5. Movement: The force of attraction between the oppositely charged electrons and protons helps the former move in the space around the nucleus in their specified orbits.
-
References
Article was last reviewed on Thursday, February 2, 2023