Brownian Motion
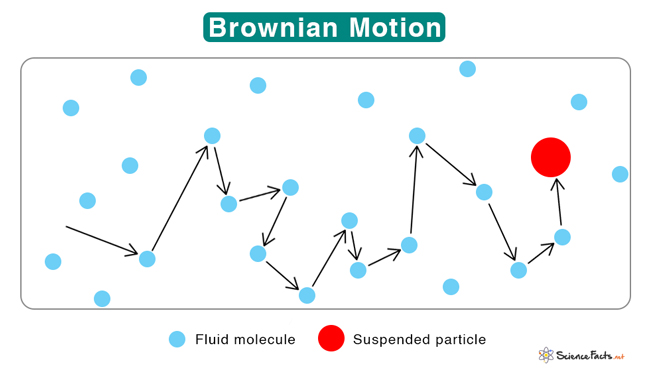
Brownian motion is the random movement of tiny particles suspended in a fluid, like liquid or gas. This movement occurs even if there is no external force. Their random motion is due to collisions. When particles collide with surrounding molecules, they move randomly, like colliding billiard balls.
Brownian motion is named after Scottish botanist Robert Brown, who first described the phenomenon in 1827. However, it was not until 1905 that Albert Einstein explained the theory in his publication on pollen movement in a liquid assisted by the liquid molecules. In 1908, French physicist Jean Perrin experimentally verified Einstein’s hypothesis, leading to the 1926 Nobel Prize in Physics.
Examples
- When light shines through a window, dust particles are observed to execute a random motion. It is due to collision with air molecules.
- Fluorescent dyes in a solution can be detected from light released by individual molecules as they move through the solution.
- Diffusion is the movement of particles from a region of a higher to a lower concentration. It can be considered a macroscopic example of Brownian motion. Diffusion of pollutants in the air, diffusion of holes through a semiconductor, and calcium diffusion through bones can be studied through Brownian motion.
Characteristics of Brownian Motion
-
References
Article was last reviewed on Saturday, February 11, 2023