Beta-Oxidation of Fatty Acid
Fatty acids are one primary energy source in tissues with high energy demand, such as the brain and heart. Like carbohydrates, fatty acids are oxidized by breaking down their long-chain acyl-CoA molecules to acetyl-CoA. The process of breaking down fatty acids in living cells is called β-oxidation.
Where Does Beta Oxidation Occur
β-oxidation is a multistep process that occurs in the cytoplasm of prokaryotic cells. In eukaryotes, it takes place in the mitochondria of the heart, skeletal muscle, and liver cells.
Activation of Fatty Acid in the Cytosol
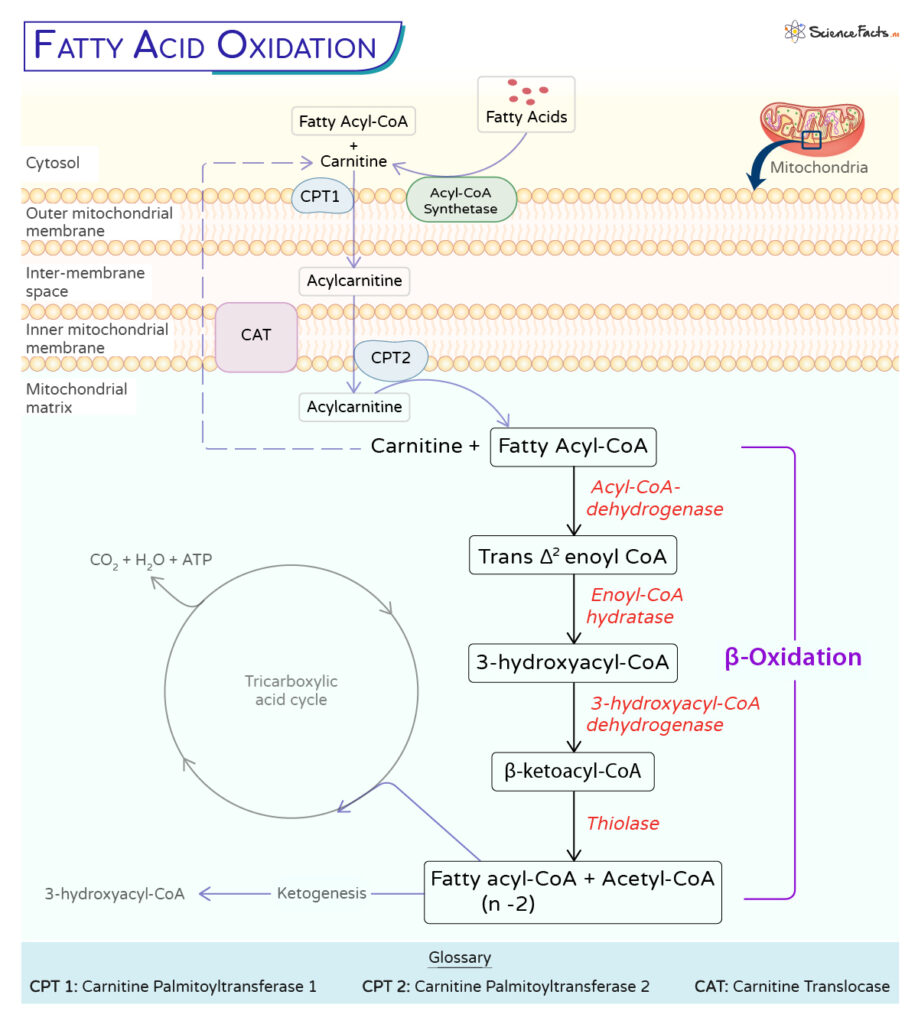
Fatty acids are synthesized in the cytosol and conjugated with coenzyme A (CoA). It leads to the formation of fatty acyl-CoA, which is catalyzed by the enzyme acyl-CoA synthetase. Each activated fatty acid utilizes one coenzyme A and one adenosine triphosphate (ATP) molecule, utilizing two high-energy bonds in one ATP molecule.
Formation of Acyl-Carnitine
However, for fatty acid degradation to occur in eukaryotes, they have to be first transported from the cytosol to the mitochondria. In the cytosol, the fatty-acyl-CoA is further modified by the carnitine palmitoyltransferase 1 (CPT1) to acylcarnitine.
Translocation into the Mitochondrial Matrix
The acyl-carnitine is then translocated across the inner mitochondrial membrane by carnitine translocase (CAT). Carnitine palmitoyltransferase 2 (CPT2) finally converts the long-chain acylcarnitine back to long-chain acyl-CoA before beta-oxidation.
Beta Oxidation in the Mitochondria
Once inside the mitochondrial matrix, the fatty acids undergo oxidation in a four-step process known as β-oxidation of fatty acid. It is so named because the β-carbon undergoes successive oxidations while removing two carbon atoms from the carboxyl end of the acyl-CoA molecule, forming acetyl-CoA.
Steps
This process involves four steps:
1. Dehydrogenation
The first step of beta-oxidation involves the oxidation of fatty acyl-CoA by the enzyme acyl-CoA-dehydrogenase, which adds a double bond between the α(C2) and β(C2) carbons of the molecule, forming trans-Δ2 enoyl CoA.
2. Hydration
Next, a water molecule is added to the trans-double bond in a reaction catalyzed by enoyl-CoA hydratase. This step forms a hydroxyl group on the β carbon, a compound known as 3- hydroxyacyl-CoA.
3. Oxidation
The hydroxyl group is then oxidized to a keto group to form beta-ketoacyl-CoA by 3-hydroxyacyl-CoA dehydrogenase, restoring the trans-double bond and generating an NADH.
4. Thiolysis
The final step involves cleaving the terminal acetyl-CoA group (β-ketoacyl-CoA) by thiolysis, releasing fatty acyl-CoA with a fatty acid chain shortened by two carbons. The enzyme thiolase catalyzes this reaction.
Thus, for each round of beta-oxidation, two carbon atoms are successively removed from the fatty acid chain. These four steps are repeated until the entire fatty acid is broken into acetyl-CoA units. Thus, it is an iterative process.
The overall equation of one cycle of beta-oxidation is:
Acyl-CoA (Cn)+ FAD + NAD+ + H2O + CoA → Acyl-CoA (Cn-2)+ FADH2 + NADH + H+ + Acetyl-CoA
Products of Beta Oxidation
The products of beta-oxidation are acetyl CoA, FADH2, NADH, and H+
The fate of the acetyl-CoA from fatty acid oxidation depends on the organism’s need. It may enter the citric acid cycle for oxidation or serve as the starting material for synthesizing fatty acids.
Once the acetyl-CoA enters the citric acid cycle, it contributes to the production of ATP through oxidative phosphorylation.
Yield of ATP
The amount of ATP synthesized from a single round of fatty acid degradation depends on the size of the fatty acid oxidized.
For example, after complete degradation, palmitic acid (a 16-carbon saturated fatty acid) forms 8 mol of acetyl-CoA. It requires the four steps to be repeated seven times, producing 7 mol of NADH and 7 mol of FADH2.
The total net yield of ATP from one molecule of palmitic acid is summarized below:
| From 1 ATP Utilized to form AMP and 2Pi | = -2ATP |
| From 8 mol of Acetyl-CoA oxidized | = 8 × 12 ATP = 96 ATP |
| From 7 mol of FADH2 oxidized | = 7 × 2 = 14 ATP |
| From 7 mol of NADH oxidized | = 7 × 2 = 21 ATP |
| Total ATP = 129 ATP |
Oxidation of 1 mol of palmitic acid produces 2,340 kcal of energy.
C16H32O2 + 23O2 → 16CO2 + 16H2O + 2,340 kcal
-
References
Article was last reviewed on Friday, February 9, 2024