Atomic Spectra
Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When an electron jumps from a higher energy level to a lower one, it emits light; when it jumps from a lower to a higher level, it absorbs light. This phenomenon occurs because an atom’s energy levels are quantized, meaning the electrons can only exist at specific energy levels and not in between.
Types of Atomic Spectra and Their Theory
Atomic spectra can be broadly classified into two types based on their appearance: continuous spectra and discontinuous (or line) spectra.
A continuous spectrum shows a smooth range of all wavelengths of light with no gaps.
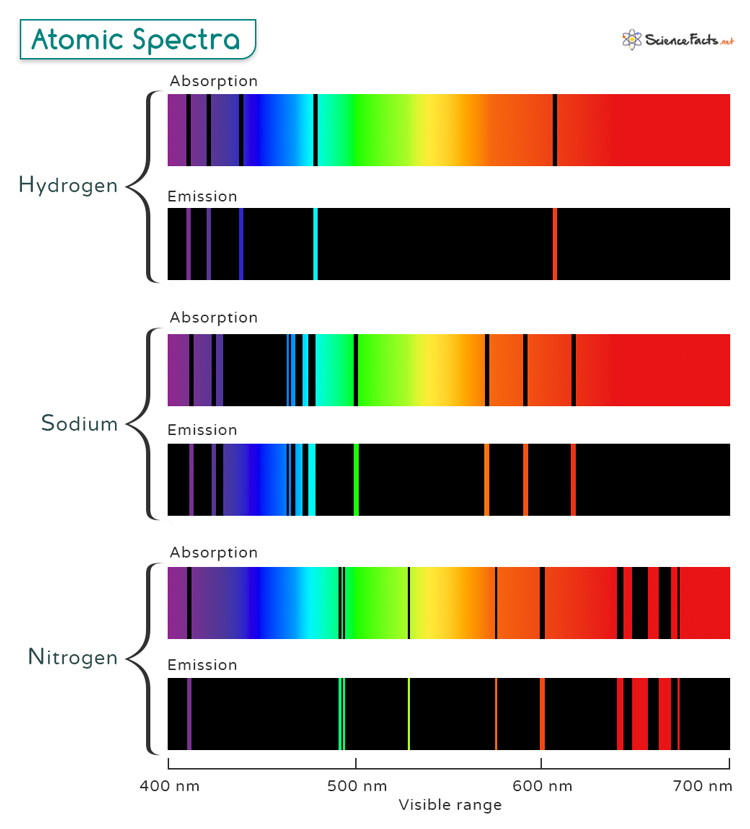
A discontinuous spectrum consists of lines of specific wavelengths superimposed over the entire range. These spectral lines provide a detailed and relevant exploration of atomic spectra. Discontinuous spectra are further classified into two types: emission spectra and absorption spectra. The image below shows the absorption and emission spectral lines of hydrogen, sodium, and nitrogen in the visible range (400 to 700 nm).
1. Atomic Emission Spectra
When atoms are exposed to a high-energy source, such as plasma or flame, their electrons are excited to higher energy levels. As these electrons return to their ground state, they release energy at specific wavelengths in the form of photons, resulting in the emission of light. This phenomenon is known as atomic emission.
2. Atomic Absorption Spectra
When a sample is atomized, typically using a flame or graphite furnace, it produces free atoms in the gaseous state. These atoms absorb light from a source that emits wavelengths characteristic of the element being analyzed. The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. This process is called atomic absorption.
To understand emission and absorption, we introduce the Bohr model of an atom. The Bohr model describes electrons orbiting the nucleus in circular paths, each with a unique energy level. When an electron transitions from a higher energy level to a lower one, it releases a photon with an energy equal to the difference between the two levels and given by the formula
Where h is Planck’s constant, c is the speed of light, and λ is the wavelength.
Equation
The Rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many chemical elements. It is particularly known for its application to the hydrogen atom, where it explains the wavelengths of the light emitted or absorbed when an electron transitions between energy levels.
The Rydberg Formula is expressed as:
Where:
- λ is the wavelength of the emitted light
- RH is the Rydberg constant, a physical constant with a value of approximately 1.097 x 10⁷ m⁻¹
- n₁ and n₂ are the initial and final energy levels of the electron, respectively
Explanation of Atomic Spectra for Hydrogen Atom
When an electron in a hydrogen atom transitions from a higher energy level (n2) to a lower energy level (n1), it emits a photon with a specific wavelength. This wavelength corresponds to the spectral line observed in the atomic spectrum. The Rydberg formula allows us to calculate these wavelengths precisely, thus explaining the observed spectral lines.
Applications to Other Elements
While the Rydberg formula was initially developed for hydrogen, it can be adapted for other elements by modifying the Rydberg constant to account for nuclear charge and electron shielding effects. This generalization helps explain the spectral lines of other hydrogen-like atoms (e.g., He+, Li2+).
Atomic Spectroscopy
Analyzing the spectral lines provides valuable information about the element’s structure and composition. The technique used for such analysis is called atomic spectroscopy. While there are several types of atomic spectroscopy, only two allow for a more straightforward and focused explanation. They are atomic emission spectroscopy and atomic absorption spectroscopy.
1. Atomic Emission Spectroscopy
Atomic emission spectroscopy (AES) is an analytical technique used to identify and quantify the elemental composition of a sample. It relies on the emission of light at specific wavelengths that are characteristic of the elements present in the sample. When the sample is excited, typically by a high-energy source like a plasma or flame, the elements emit light at their characteristic wavelengths.
AES can be used for both qualitative analysis (identifying which elements are present) and quantitative analysis (determining how much of each element is present). It is commonly used in environmental monitoring, materials science, and food and pharmaceutical analysis. One key advantage of AES is its ability to detect and measure trace elements, making it a valuable tool for various industries. Additionally, AES is a relatively simple and cost-effective technique.
2. Atomic Absorption Spectroscopy
Atomic absorption spectroscopy (AAS) is a widely used analytical technique for quantifying trace elements in various samples. It relies on the principle of light absorption by free atoms in the gaseous state. AAS involves atomizing the sample through a flame or furnace to convert the analyte elements into free, unexcited atoms in the ground state. These atoms then absorb light at specific wavelengths, with the amount of absorption being proportional to the element’s concentration, as per the Beer-Lambert law.
AAS is highly sensitive and selective, enabling the analysis of trace elements at parts-per-million or parts-per-billion levels. It is widely used in environmental monitoring, food and beverage analysis, clinical diagnostics, and materials science.
Applications
- In analytical chemistry, elemental spectral signatures are used to identify and quantify material compositions. Atomic absorption/emission spectroscopy is invaluable for studying samples in fields such as metallurgy, geology, and environmental monitoring.
- In materials science, atomic spectroscopy provides insights into the electronic structure and bonding of materials, which is crucial for developing new advanced materials.
- Astrophysicists use atomic spectroscopy to determine the composition, temperature, and properties of celestial bodies, helping to shed light on the evolution of the universe.
- Studying atomic spectra benefits plasma physics, as the emission patterns of ionized gases can be used to diagnose the properties of high-temperature plasmas.
- Atomic spectroscopy is essential in environmental monitoring and medical diagnostics by detecting specific elemental concentrations.
-
References
Article was last reviewed on Thursday, August 22, 2024