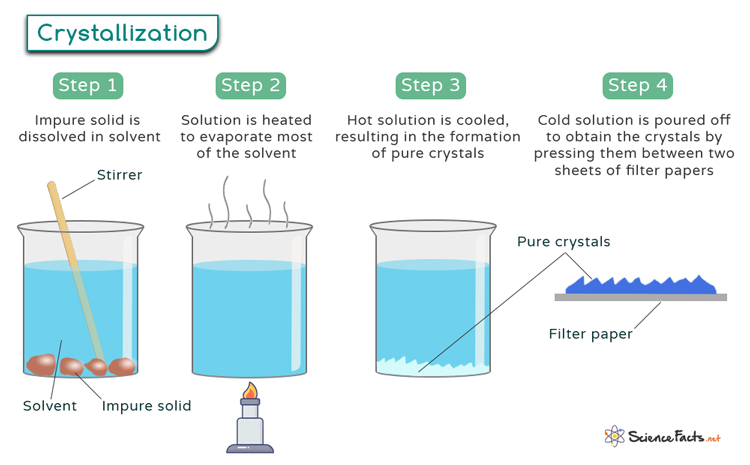
Crystallization
Crystallization, a fundamental process in nature and industry, is pivotal in forming structured solids from liquids or gases. It involves the orderly arrangement of atoms, ions, or molecules into a highly organized, repeating pattern known as a crystal lattice.
In other words, crystallization refers to transforming from a disordered, amorphous state to a well-defined, ordered crystalline structure. This process occurs through the formation of crystals, which are three-dimensional arrangements of atoms or molecules characterized by a repeating pattern. Crystals exhibit unique geometric shapes and possess distinct physical and chemical properties, making them essential in various scientific and industrial applications.
Crystallization Process
The crystallization process is guided by three fundamental principles: nucleation, growth, and aggregation.
1. Nucleation
Nucleation marks the inception of the crystallization process, where individual molecules, ions, or atoms assemble into clusters, forming the initial nuclei. This critical phase sets the stage for the subsequent growth of crystals. The nucleation process can occur spontaneously, but it is often influenced by external factors such as temperature and the presence of impurities.
2. Growth
Following nucleation, the crystalline nuclei undergo growth as additional molecules join the evolving structure. This growth phase is characterized by continuously adding building blocks to the crystal lattice, expanding the crystal’s size and complexity. The rate and manner of growth significantly impact the final crystal’s properties, making it a crucial aspect of the crystallization process.
3. Aggregation
As crystals grow, they may encounter each other, leading to the aggregation of multiple crystalline structures. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Understanding the aggregation process is vital for controlling the desired crystal characteristics in laboratory and industrial settings.
Examples of Crystallization
Crystallization is a ubiquitous phenomenon, evident in both natural and industrial settings, showcasing the intrinsic beauty and utility of this transformative process.
Natural Crystallization
1. Formation of Snowflakes
One of the most enchanting examples of natural crystallization is the formation of snowflakes. As water vapor in the atmosphere undergoes cooling, it undergoes crystallization, with individual ice crystals forming unique and intricate hexagonal patterns. The diversity in snowflake shapes results from variations in temperature and humidity during their descent, highlighting the exquisite beauty that arises from the fundamental principles of natural crystallization.
2. Geological Crystal Formation
The Earth’s geological processes produce many crystalline structures, from quartz in granite to diamonds formed under high-pressure conditions. Over geological time scales, minerals undergo crystallization, leading to the creation of stunning crystals and gemstones. The diversity of minerals and the conditions under which they form contribute to the vast array of crystal formations found in rocks and geological formations.
Industrial Crystallization
1. Salt Production
Crystallization plays a pivotal role in the production of salt. This process typically involves the evaporation of seawater or brine, leading to salt crystallization. The controlled conditions, including temperature and concentration, govern the size and quality of the salt crystals produced. This method has been employed for centuries and is a primary means of obtaining salt for various industrial and culinary purposes.
2. Sugar Crystallization
The sugar industry relies heavily on crystallization for the production of refined sugar. Sugar beet or sugarcane juice is processed, and impurities are removed to create a supersaturated solution. By carefully controlling temperature and seed crystal addition, sugar molecules crystallize, forming the familiar granulated sugar. The size of sugar crystals can be tailored to meet specific industrial and consumer needs.
3. Pharmaceutical Crystallization
In the pharmaceutical industry, crystallization is a crucial step in drug manufacturing. Pharmaceutical compounds are often isolated and purified through crystallization, ensuring the production of high-purity drugs. Controlling the crystallization conditions allows for generating pharmaceutical crystals with desired properties, impacting factors such as bioavailability and stability.
Factors Influencing Crystallization
Several factors influence the crystallization process. They are:
1. Temperature
Temperature stands as a paramount factor influencing crystallization. The rate at which molecules come together and form crystals is highly sensitive to temperature variations. Precise temperature control during crystallization is essential for achieving desired crystal sizes, purities, and morphologies. Temperature modulation can also influence nucleation, affecting the number and size of crystals formed.
2. Solvent Selection
The choice of solvent significantly impacts crystallization outcomes. Solvents influence the solubility of the solute, affecting nucleation and growth. The solvent’s ability to dissolve the solute at elevated temperatures and then allow controlled precipitation during cooling plays a crucial role. Solvent selection is a strategic decision in optimizing crystallization processes for specific applications.
3. Impurities
The presence of impurities can profoundly impact crystallization, influencing nucleation, growth, and crystal quality. Impurities may act as nucleation sites, altering the number and size of crystals formed. Their effects on crystal growth can result in variations in crystal size and purity. Therefore, meticulous attention to impurity control is essential in achieving reproducible and high-quality crystalline products.
Applications
The significance of crystallization reverberates across various fields, influencing everything from basic research to industrial processes. Some applications are:
- Production of high-purity chemicals through crystallization for different industrial applications
- Separation and purification processes to isolate specific compounds from complex mixtures
- Refining of sugar and salt through crystallization processes to meet quality and purity standards
- Drug crystallization for formulation, ensuring the production of pharmaceuticals with consistent quality and efficacy.
- Semiconductor manufacturing, where controlled crystallization is essential for the fabrication of electronic components.
- Crystal growth for optoelectronics influences the development of materials for devices like lasers and solar cells.
- Removal of impurities from water through crystallization contributes to producing high-quality drinking water.
- Crystallization-based methods for the recovery of valuable minerals from industrial wastewater
- Controlled crystallization synthesizes nanomaterials for various applications, including catalysis and drug delivery.
- Crystallization is used in dyeing and finishing textiles for improved color fastness and fabric properties.
-
References
Article was last reviewed on Thursday, February 15, 2024