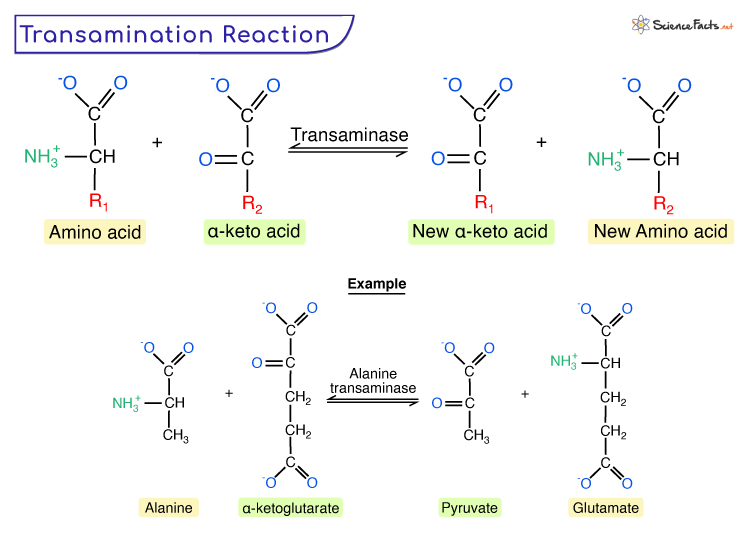
Transamination
Transamination is a biochemical process that involves the transfer of an amino group (NH2) from one amino acid (except lysine, proline, and threonine) to a keto acid (without an amine group), producing a new amino acid and a corresponding new keto acid. It is thus a reversible amination and deamination.
Given is the generic form of transamination reaction.
During transamination, the amino group is usually transferred to the keto carbon of pyruvate, oxaloacetate, or α-ketoglutarate, converting the α-keto acid to alanine, aspartate, or glutamate. It is accomplished by enzymes known as transaminases or aminotransferases, having pyridoxal phosphate as a coenzyme (a derivative of vitamin B6).
An aminotransferase may be specific to an amino acid or particular to any member of a group of similar amino acids. Alanine aminotransferase and aspartate aminotransferase are two common types of aminotransferases.
Where Does Transamination Occur
Transamination occurs in the mitochondria and cytoplasm of eukaryotic cells and requires the synthesis of non-essential amino acids from essential amino acids obtained de novo (from dietary intake).
Aminotransferases are widely distributed in human tissues, including heart muscle, liver, skeletal muscle, and kidney.
Mechanism of Transamination
Transamination reaction occurs in two steps:
Step 1: The α-amino group of an amino acid is first transferred to the enzyme, producing the corresponding α-keto acid and the aminated enzyme.
Step 2: The amino group is then transferred to the keto acid acceptor, forming the amino acid, regenerating the enzyme.
Thus the product of transamination depends on the availability of α-keto acids. In the overall reaction, the amino acid transfers its amino group to pyridoxal phosphate and then to the keto acid by forming the intermediate, pyridoxamine phosphate.
Examples of Transamination
Some common transamination reactions are tabulated below:
| Examples | Enzymes | Product |
|---|---|---|
| 1. Alanine to Glutamate Transamination | Alanine transaminase | Pyruvate and glutamate |
| 2. Aspartate-α-Ketoglutarate Transamination | Aspartate transaminase | Oxaloacetate and glutamate |
| 3. Glutamate-α-Ketoglutarate Transamination | Glutamate aminotransferase | α-ketoglutarate and glutamate |
| 4. Serine-Pyruvate Transamination | Alanine aminotransferase | Alanine and hydroxypyruvate |
| 5. Phenylalanine-Pyruvate Transamination | Phenylalanine transaminase | Phenylpyruvate and alanine |
| 6. Tyrosine to α-Ketoglutarate | Tyrosine aminotransferase | p-Hydroxyphenylpyruvate + glutamate |
| 7. Asparagine-Oxaloacetate Transamination | Asparagine aminotransferase | Aspartate and α-ketoglutarate |
| 8. Histidine to α-Ketoglutarate Transamination | Histidine transaminase | Imidazolepyruvate and glutamate |
Importance of Transamination
Transamination plays a vital role in diverse biological processes that helps to maintain the proper functioning of living organisms.
Synthesis and Recycling of Amino Acids
By facilitating the transfer of amino groups from one amino acid to another, transamination contributes to the synthesis of different amino acids.
Moreover, it is a crucial component of the amino acid recycling process. When proteins are degraded, or amino acids are in excess, transamination allows the conversion of excess amino acids into forms that can be excreted as waste. It thus maintains nitrogen balance within the body, preventing the buildup of toxic ammonia.
Energy Production
Transamination reactions generate ATP, the cell’s energy currency. It also helps to convert amino acids to intermediates that can enter metabolic pathways like the citric acid cycle and glycolysis.
Neurotransmitter Synthesis
Several amino acids involved in transamination play crucial roles in neurotransmitter synthesis. For example, glutamate and aspartate are synthesized through transamination.
Apart from the above functions, transamination also helps to detoxify drugs and xenobiotics and has implications in enzyme assays in medical diagnosis.
-
References
Article was last reviewed on Tuesday, August 29, 2023