Surface Tension
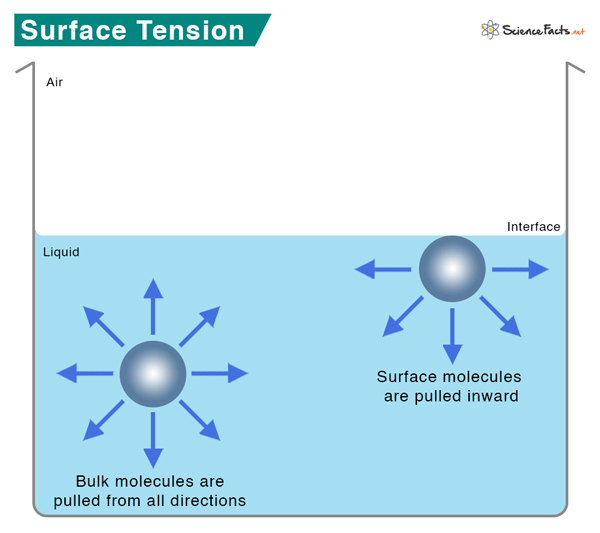
Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the sides and bottom. As a result, the liquid surface forms a “skin” with minimum surface area and energy.
What Causes Surface Tension
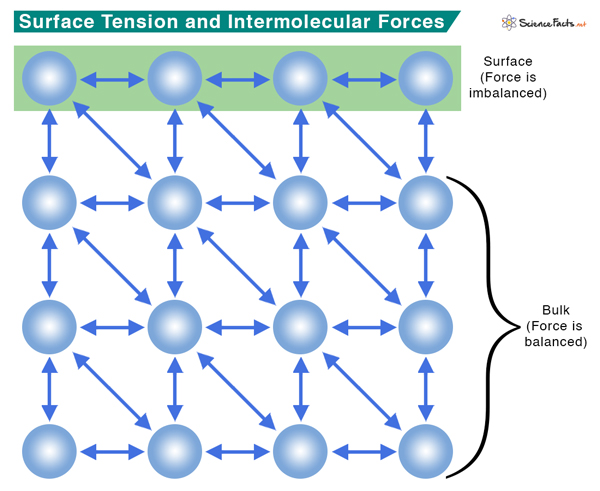
Intermolecular forces like hydrogen bonding and Van der Waals forces hold the molecules together. Within the body of a liquid, molecules do not experience any force because the interactive forces from the neighboring molecules cancel out. On the surface, the molecules are attracted from the sides and bottom. No water molecules exist on the top; hence, no attractive forces from the top. As a result, the surface molecules contract and resist stretching and rupture. The surface is under tension, which explains the origin of the word surface tension.
Units
Since surface tension refers to the interactive force, it is quantified as the force per unit length or energy per unit area. Therefore, its SI unit is Newton per meter of N/m. Its cgs unit is dyne/cm. In terms of energy units, they are Joules per square meter (J/m2) in the SI system and ergs/cm2 in the cgs system.
Surface Tension of Water
Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by two hydrogen atoms. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. As a result, molecules are held together by strong hydrogen bonding. Energy is required to break these intermolecular bonds. Since water has a high surface tension, much energy is required to separate its molecules. The higher a liquid’s polarity, the higher the surface tension.
Cohesive and Adhesive Forces
Surface tension can explain cohesive and adhesive forces. Cohesive forces hold the liquid body with minimum surface area. Adhesive forces are responsible for the body to spread out. The liquid will hold its shape if the cohesive forces exceed adhesive forces. An example is mercury, whose surface tension at 25 °C is 485 mN/m. Suppose the cohesive forces are less than the adhesive forces. In that case, the liquid will spread out, thus, maximizing its surface area. This process is called wetting. Water has a surface tension of 72 mN/m and can easily wet surfaces.
The shape of water and mercury meniscuses in a test tube depends on cohesive and adhesive forces. The meniscus of water is upward or concave because water wets the surface and creeps up the side. On the other hand, the meniscus of mercury is downward or concave because mercury does not wet glass. The cohesive force is strong enough to form a drop.
Examples of Surface Tension
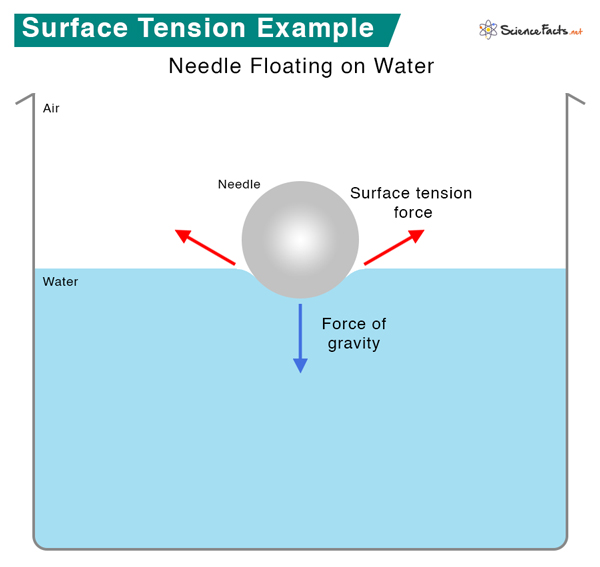
1. Objects floating on water – Objects can float on the water’s surface if they do not rupture the “skin” and separate the molecules. The surface behaves like an elastic membrane that can resist the object’s weight. Small insects like water striders can walk on water because their lightweight bodies cannot penetrate the surface. It shows that surface tension is important to life. The surface tension of water can also support needles and razor blades.
2. Jaundice test – Powdered sulfur is spread over urine. It will remain on the surface if urine is free of bile. However, bile reduces the surface tension of urine. If bile is present in urine, the sulfur powder will sink.
3. Hot water, soaps, and detergents – Hot water has a lower surface tension than cold water. Therefore, it is an excellent “wetting” agent. Soaps and detergents further reduce surface tension so water can enter pores and soiled parts. Therefore, detergents added to hot water make them perfect for washing clothes.
4. Liquid droplets – They are round because the cohesive forces of the surface layer pull into a spherical shape. For this reason, surface tension is an important property to study.
5. Surface tension disinfectants – Disinfectants are usually solutions of low surface tension. Therefore, they can spread out on the cell walls of bacteria and disrupt them.
6. Tents – Tents used for camping are made of materials that prevent rainwater from penetrating inside. The surface tension of water bridges the pores in the finely woven material. However, touching the tent will break the surface tension, seeping water through the tent.
Surface Tension Values
The following table gives the surface tension values of different liquids at 20 °C.
| Substance | Surface Tension at 20 °C (mN/m) |
|---|---|
| Acetone | 25.2 |
| Benzene | 28.88 |
| Chloroform | 27.5 |
| Ethanol | 22.1 |
| Glycerol | 64 |
| Isopropanol | 23 |
| Methanol | 22.7 |
| Toluene | 28.4 |
| Water | 72.8 |
| Mercury | 485.8 |
FAQs
Ans. Increasing the temperature increases the kinetic energy of the molecules. As the molecules get agitated, they lose the efficiency of intermolecular attraction. Due to reduced cohesive forces, the surface tension decreases as temperature increases.
Ans. No, surfactants are designed to reduce surface tension. They allow liquid to wet surfaces.
Ans. The presence of soluble particles in water may increase or decrease surface tension. The surface tension will decrease if the impurities are less soluble (for example, camphor). On the other hand, the surface tension will increase if the impurities are more soluble (for example, salt).
-
References
Article was last reviewed on Friday, October 6, 2023