Intermediate Filaments
Intermediate filaments (IFs) are one of the primary components of the cell cytoskeleton, along with microtubules and microfilaments. They are made of multiple strands of fibrous proteins wound together, each consisting of amino acids arranged in a chain.
IFs are dynamic, motile elements that interact with a range of cellular proteins to function. They are the most stable cytoskeletal component and thus provide mechanical strength to cells and tissues.
Where are Intermediate Filaments Found
They form an extensive network in the cytoplasm of most animal cells, extending from a ring surrounding the nucleus to the plasma membrane. However, IFs are absent in plants and fungi.
Structure
IFs are so named because they have a diameter of about 10 nm. They are thicker than actin filaments (about 7 nm) and thinner than microtubules (about 25 nm) or muscle myosin filaments.
Despite considerable differences in size and amino acids sequence, the various intermediate filament proteins share a typical structural organization. Each IFs monomer consists of a central α-helical rod domain of approximately 310 amino acids, flanked by amino- and carboxy-terminal domains. The central domain varies among intermediate filament proteins in size, sequence, and secondary structure.
Types of Intermediate Filament Proteins
IFs are the most diverse group among all cytoskeletal elements made of various proteins expressed in different types of cells. At least 50 different intermediate filament proteins have been identified so far. They are classified into different groups based on similarities between their amino acid sequences. They are:
- Type I and II: They consist of two groups of keratins, each made of 15 different proteins, which are found to express in epithelial cells.
- Type III: They include vimentin and desmin. Vimentin is found in different cell types, such as fibroblasts, smooth muscle cells, and white blood cells, whereas desmin expresses specifically in muscle cells. A third and fourth type III IFs are found in glial cells and neurons of the peripheral nervous system.
- Type IV: They include the three neurofilaments (NF) proteins, named NF-L (L for light), NF-M (M for medium), and NF-H (H for heavy). These proteins are found in many mature neurons, particularly in motor neurons’ axons.
- Type V: They are lamins found in most eukaryotic cells. They are not part of the cytoskeleton. Instead, they are components of the nuclear envelope.
- Type VI: They include beaded filaments such as filensin and phakinin.
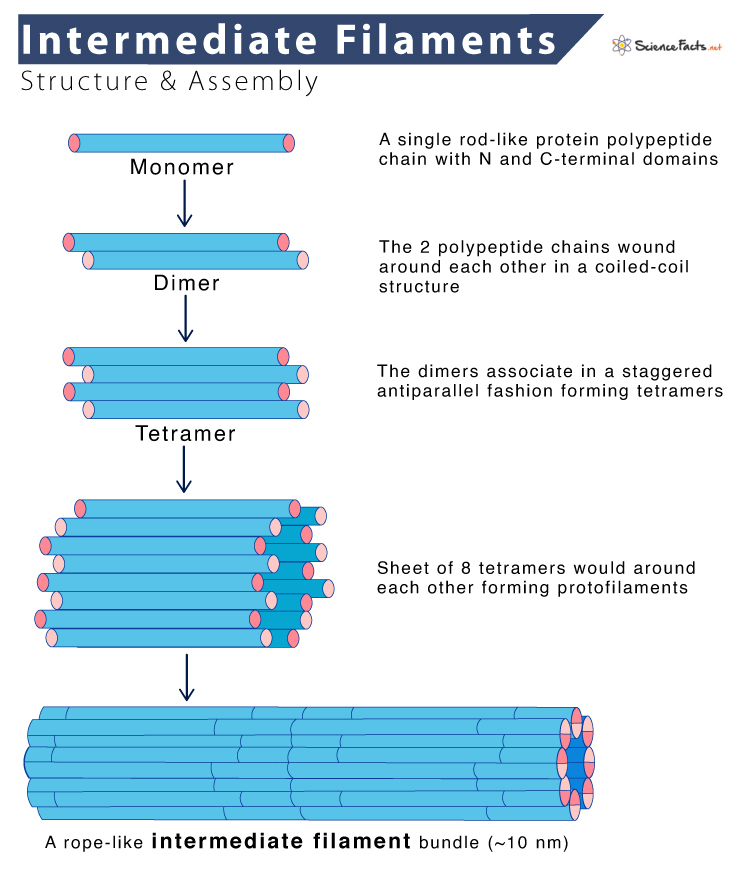
Intermediate Filaments Assembly
- It starts with the formation of dimers. The central rod domains of two polypeptide chains are wound around each other in a coiled-coil structure, similar to the assembly of myosin II heavy chains.
- The dimers then associate in a staggered antiparallel fashion to form tetramers, which can assemble end to end, forming protofilaments.
- The final intermediate filament contains approximately eight protofilaments wound around each other in a ropelike structure.
Thus, in contrast to actin filaments and microtubules, intermediate filaments do not have distinct plus and minus ends and thus lack polarity.
Functions
The primary function of IFs is to provide structural support to the cell. They also act as a mechanical stress absorber for the entire cell cytoskeleton. The tight association between their protofilaments helps in:
- Regulating cell shape in association with some other proteins such as cadherins and integrin
- Anchoring the nucleus and other organelles in place and thus creating supportive scaffolding inside the cell
- Integrating the cytoskeleton
- Regulating some key signaling pathways
- Moving proteins to specific domains of polarized cells such as Sertoli cells
- Providing high tensile strength in durable structures such as hair, scales, and fingernails
- Maintaining the alignment of myofilaments and sarcomeres in skeletal muscles
Apart from structural functions, IFs also have a significant role in cell-type-specific functions and regulate gene expressions and mitosis.
FAQs
Ans. Yes, sperm cells or spermatozoa contain intermediate filaments of vimentin type.
Ans. No, collagen is not an intermediate filament.
-
References
Article was last reviewed on Friday, February 17, 2023