Electron Cloud
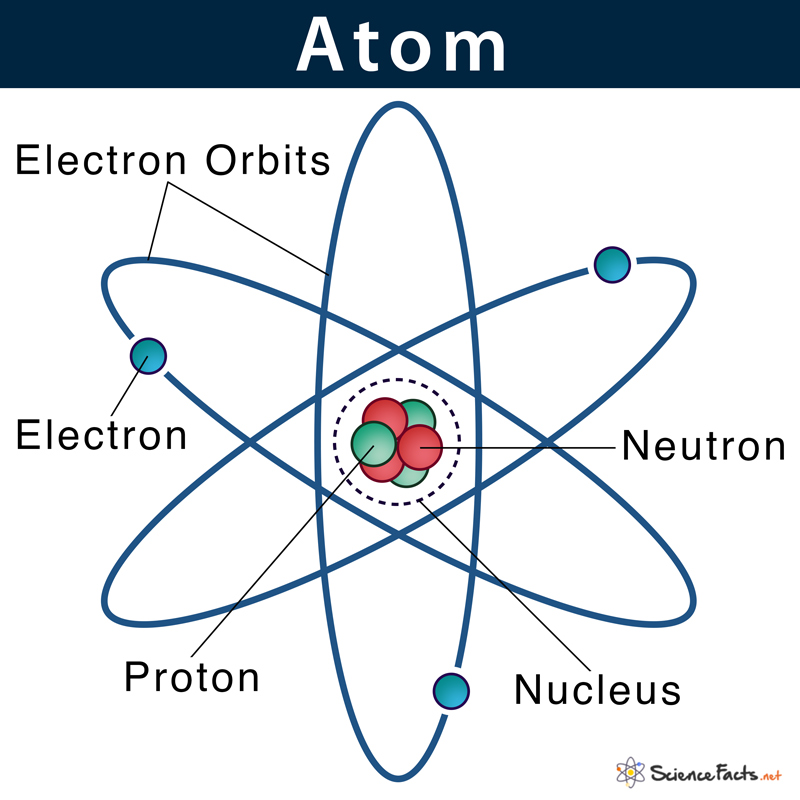
In 1913, Niels Bohr proposed the shell model of an atom. He postulated that the subatomic particles, protons, and neutrons reside within the atomic nucleus, whereas the electrons revolve around the nucleus in specific orbits or shells.
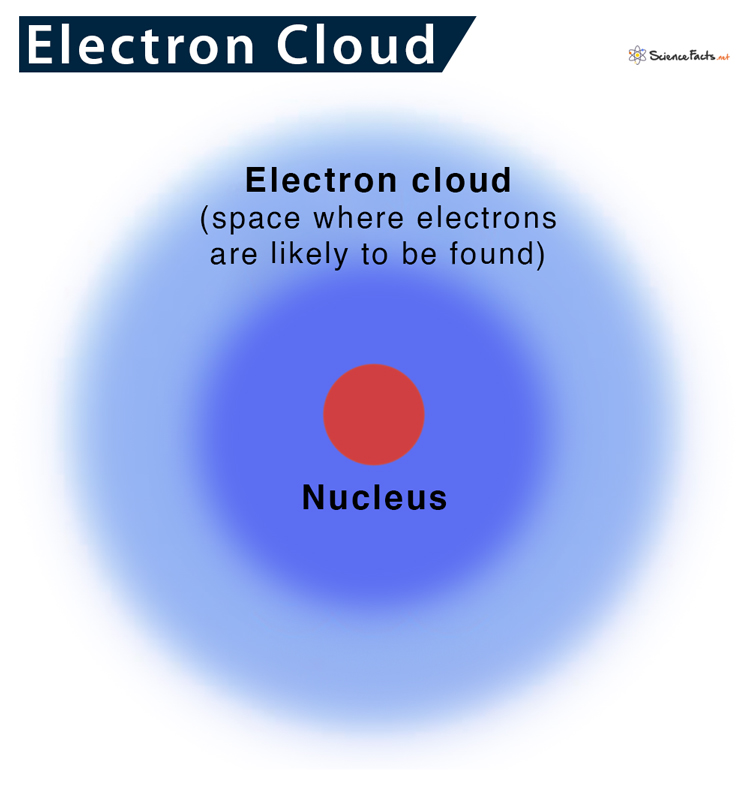
Later in 1925, some scientists put forward another theory, regarding the position of electrons in an atom, named the electron cloud model.
What Is An Electron Cloud?
As stated, previously electrons were thought to occupy some fixed orbits around the nucleus. However, as more research was done on quantum mechanics, it was found that electrons do not remain confined within any fixed orbits. Instead, the exact location of an electron cannot be specified.
Around 1925, Erwin Schrödinger and Werner Heisenberg were trying to find a way to properly describe the uncertainty of the position of electrons in an atom. They developed an equation that can determine where an electron is most likely to be. Based on the calculations, they identified the probable regions around the nucleus, where there are chances of finding an electron. They named these electron-dense regions as orbitals. So, it can be concluded that electrons do not revolve around the nucleus in perfect spheres but rather a dense cloudy region of probability.
Here is an easy way to understand the concept of electron cloud theory:
Observe a fan running at high speed. Can you see its individual blades? No. At that time, the fan blades move so fast that you cannot clearly see the individual blades. You cannot tell where a particular blade is at any given moment. Nevertheless, the blades are certainly within that area only, but you cannot point out a particular position.
Similarly, electrons also move around the nucleus at a very high speed. So, determining a particular position of an electron is difficult. So, we can never tell exactly where they are. They can be anywhere, encircling the region around the nucleus, thus giving an illusion of cloud.
-
References
Article was last reviewed on Thursday, February 2, 2023