Electron Shells
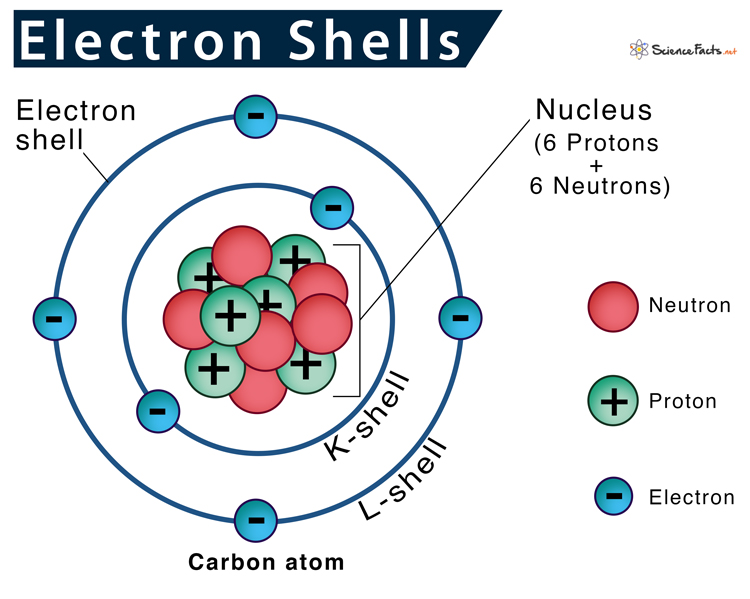
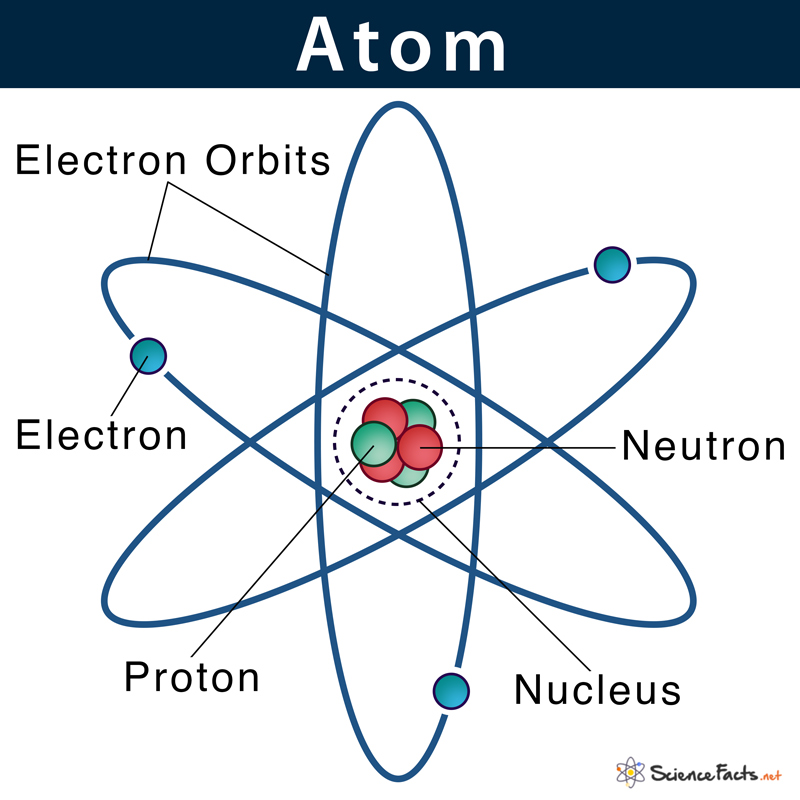
Electron shells or energy levels are the outermost part of an atom surrounding the atomic nucleus. It can be thought of as paths followed by electrons around the nucleus.
Electron Shells in the Rutherford-Bohr Model
According to the Rutherford-Bohr model of atomic structure, each electron shell has a specific identity based on its distance from the nucleus, named the principal quantum number (n). Principal quantum numbers start with 1 and increase by 1 every time, so the first four energy levels have their n values as 1, 2, 3, 4, and so on. So, the higher the principal quantum numbers higher the energy level of the shell.
They are also labeled as K, L, M, N, O, P, and Q going from the innermost shell to the outermost one. So, the K-shell is closest to the nucleus, and Q-shell is the farthest.
How Many Electrons Are in Each Shell
Each successive electron shell can only hold a certain number of electrons. Higher energy shells can hold more electrons. The first shell can only contain two electrons, the second eight and the third eighteen. Electrons in the same shell have the same amount of energy.
The general rule stating the number of electrons present in a shell is 2n2, where ‘n’ stands for the number of shells; for example, K-shell = 1, L-shell = 2. First, the innermost shell is filled, and then the later ones.
The table below shows the configuration of the electron shell.
| Electron shell | Maximum number of electrons (2n2) |
|---|---|
| 1st ( K-shell) | 2(1)2 = 2 |
| 2nd ( L-shell) | 2(2)2 = 8 |
| 3rd ( M-shell) | 2(3)2 = 18 |
| 4th ( N-shell) | 2(4)2 = 32 |
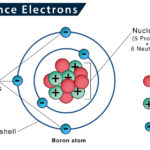
Valence Shell and Valence Electrons
The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical reactions. The electrons present in these shells are referred to as valence electrons.
For instance, in a noble gas (except helium), an atom has eight valence electrons in its outermost shell, achieving an octet or stable state. An atom can roam freely or not participate in any reaction in this state. As a result, atoms other than noble gases tend to form bonds with other atoms to have eight electrons in their outermost shell. They do so by forming an electrovalent, covalent, or coordination bonding.
Electron Sub-shells
Each electron shell has one or more sub-levels, called subshells. The subshells are represented by the letters like s, p, d, f, g, h, and i.
For example, the first (K) shell has one subshell, called 1s; the second (L) shell has two subshells, called 2s and 2p; the third shell has 3s, 3p, and 3d; the fourth shell has 4s, 4p, 4d and 4f; the fifth shell has 5s, 5p, 5d, and 5f and 5g.
Each subshell is constrained to hold 4ℓ + 2 number of electrons at most, where ℓ is the azimuthal quantum number. The azimuthal quantum numbers are 0, 1, 2, 3, 4, 5, 6, corresponding to the subshells s, p, d, f, etc.
The table below gives the maximum number of electrons each subshell can hold:
| Electron Sub-shell | Maximum number of electrons (4ℓ + 2) |
|---|---|
| s | (4×0) + 2 = 2 |
| p | (4×1) + 2 = 6 |
| d | (4×2) + 2 = 10 |
| f | (4×3) + 2 = 14 |
| g | (4×4) + 2 = 18 |
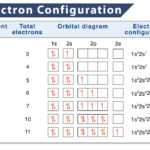
Subshell Energy Levels and Filling Order
Each subshell has a different energy level, like each electron shell’s own energy level. S sub-shells have the lowest energy level, followed by p, d, and f.
When electrons are filled in the subshells, they proceed from subshells of lower energy to higher energy, following the Aufbau principle. The electrons follow the ‘n + ℓ’ rule, also known as the Madelung rule. Here, ‘n’ stands for the principle quantum number, whereas ‘ℓ ‘represents the azimuthal quantum number. Subshell having a lower ‘n + ℓ’ value fills before those with higher ‘n + ℓ’ values. In the case of equal n + ℓ values, the subshell with a lower n value is filled first.
Electron Orbitals
When an electron shell is divided into one or more subshells, they are simply sets of one or more orbitals. Each letter of the sub-shells indicates a different shape. For example, subshells have a single, spherical orbital. In contrast, p subshells contain three dumbbell-shaped orbitals at right angles to each other. Subshells d and f have more complex shapes and contain five and seven orbitals, respectively.
Each orbital contains a maximum of 2 electrons, although they can have fewer than two. The different sub-shells also have different numbers of orbitals, influencing how many electrons they can hold. S sub-shells only have one orbital, p sub-shells have three, and the d sub-shell has five. Electron configuration is the simplest and shortest way of showing how electrons are filled up in each orbital of an atom.
For example, the electron configuration of carbon atom is written as 1s2,2s2,2p2 having 2 electrons in the K shell and 4 in the next L shell. Neon, on the other hand, has 10 electrons distributed as 2 each in 2s, and 3 p orbitals, with configuration 1s2,2s2,2p6.
Electron Spin
The electrons in an orbital have opposite spins. The direction of one electron is always spin up, while the other is in a spin-down position.
Orbital Energy
Orbitals in the same sub-shell contain the same energy. For example, both the electrons in the 3s subshell have the same energy level.
Periodic Table with Electron Shells
Elements on Earth are organized in a tabular form according to their chemical and physical properties. Presented by Dmitri Mendeleev in 1869, the table places elements into columns (groups) and rows periods on the basis of their atomic number and atomic mass.
The number of electron shells in an atom and the distribution of electrons in the shell determine the chemical reactivity of the atom and its periodic table position. Two atoms with complementary electron patterns can react and form a chemical bond, creating a molecule or compound.
-
References
Article was last reviewed on Thursday, May 9, 2024