Enzyme
Any substance that speeds up a biochemical reaction without being a reactant is called a catalyst. The catalysts for biochemical reactions in living systems are known as enzymes. They are thus known as biological catalysts or biocatalysts.
What are Enzymes
Enzymes are protein macromolecules that are necessary to initiate or speed up the rate of chemical reactions in the bodies of living organisms. The molecules on which enzymes act are called substrates, and the substance formed is called the product.
They are found in all living cells that vary in type based on the function it performs. Enzymes help in the process of digestion, blood clotting, and hormone production.
Are All Enzymes Proteins
Almost all known enzymes are proteins. Previously it was believed that all enzymes are chemically protein in nature. Then, certain nucleic acids known as ribozymes are also found to have catalytic properties. Students study protein-based enzymes when they learn about this group of organic biomolecules. The reason is that very little is known about ribozymes.
Components
Proteins are composed of long amino acids chains that are held together by peptide bonds. Some enzymes are made up of only one chain of amino acids, while most others are made of multiple chains.
Cofactors
Some enzymes require a non-protein part for their functioning, known as cofactors. Cofactors are essential for the functioning of the enzyme. An enzyme devoid of a cofactor is an apoenzyme, while an enzyme with its cofactor is called a holoenzyme.
There are three types of cofactors:
1) Prosthetic Groups: They remain tightly bound to an enzyme all the time. Example – FAD
2) Coenzyme: They bind to an enzyme only during catalysis. Example – NAD+
3) Metal Ions: Certain enzymes require a metal ion at their active site for catalysis. They form a coordinate bond with the enzyme. Example – Zn2+
Structure: What are They Made of
Each enzyme is made up of a unique chain of amino acids and has a unique shape. It can assume any of the three types of structure – primary, secondary, and tertiary structure. Enzymes made of amino acids that are arranged in a polypeptide chain produce the primary structure. The formed amino acid chain is called a polypeptide.
The protein folds upon itself when the hydrogen in the (NH2) group and the oxygen in the (COOH) group forms a hydrogen bond. It results in the folding of the protein in the 2-dimensional plane. There are two types of secondary structures – α-helix and β-sheet.
As a result of folding the 2-D linear chain in the secondary structure, the protein can fold up further, which helps it gain a 3-D structure. This formation is the tertiary structure of the protein.
Properties
General
- Protein in nature except for ribozyme
- Activity depends on several environmental factors such as temperature, pH, and concentration of substrate and products
- Initiate chemical reaction(s)
- Accelerate the rate of chemical reaction(s). The rate of an enzyme-catalyzed reaction usually is (103 – 108) times faster than an uncatalyzed reaction.
Apart from the general properties, enzymes have four more essential properties. They are given below:
Catalytic
- Have excellent catalytic power
- Highly capable; a small quantity of an enzyme can catalyze a large quantity of a specific substrates.
- It remains unaltered at the end of the reaction
- The turnover number ranges between 0.5 to 600000
Specificity
- Specific to a particular type of reaction. A particular enzyme catalyzes a particular reaction binding to a particular substrate.
Reversibility
- Most reactions are reversible. The reversibility depends on the requirement of the cell
- In some cases, there are separate enzymes for forwarding and backward reaction
- Some reactions are not reversible
Sensitivity to Temperature and pH
- Highly sensitive to heat, temperature, and pH
- Activity is maximum within a specific range of temperature and pH ranges. Below which they have reduced activity and above which they get denatured and thus lose their activity.
When and How does an Enzyme Work to Catalyze Reactions
Enzymes work by lowering the activation energy – the amount of energy needed for the reaction to begin. Enzymes work by binding to reactant molecules, holding them so that the formation and breaking of bonds during the process can take place readily.
Enzymes do not change the free energy of the reactants and the products and thus do not affect a reaction’s ∆G value. Instead, they work by lowering the transition state, an intermediate state in the reaction. They also keep the equilibrium constant (Keq) same throughout the reaction.
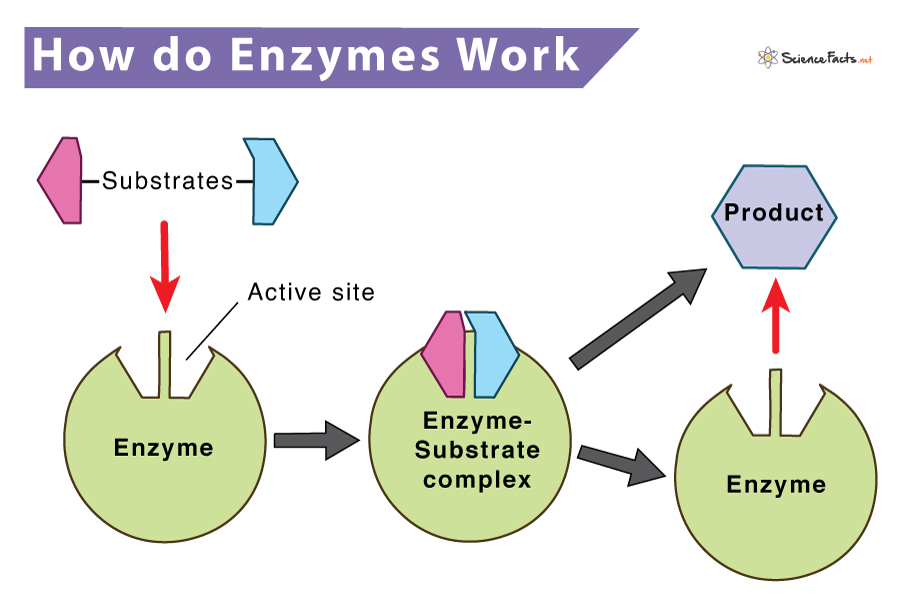
While catalyzing a chemical reaction, an enzyme binds to one or more reactant molecules. These molecules are enzymes substrates. In some reactions, one substrate molecule breaks to form multiple products. In contrast, in others, two substrate molecules join to form one large molecule. The part of the enzyme where the specific substrate binds is called the active site. The basic mechanism is divided into two steps, as shown below:
Step 1:
Enzyme (E) + Substrate (S) <—–> Enzyme-Substrate complex (ES)
Step 2:
Enzyme-Substrate complex (ES) <—–> Enzyme (E) + Product (P)
The above two steps can be combined as follows to give the complete reaction.
Overall Reaction:
Enzyme (E) + Substrate (S) <—–> Enzyme-Substrate complex (ES) <—–> Enzyme (E) + Product (P)
The amino acids present in the active site of the enzyme give the enzyme a definite shape. The shape uniquely determines its substrate and helps it to bind and form the enzyme-substrate complex. The enzyme then converts the bound substrate to a product with itself remaining chemically unchanged.
How do They Speed up Reactions
As stated above, enzymes work by decreasing the activation energy, like all catalysts. They generally lower the activation energy by reducing the energy needed for reactants to react during a reaction. Lesser the activation energy of a reaction, the faster the rate of the reaction. They do so in the following ways:
- Bring the reactants together such that they do not need to expend energy moving about to collide.
- Position the reactants correctly so that they do not have to overcome intermolecular forces that would typically push them apart
- Change the pathway so that the reaction can occur by the pathway with lower activation energy.
What Happens to an Enzyme after a Biochemical Reaction
After the reaction, the products formed are released from the active site of the enzyme. The enzyme remains unaltered at the end of the reaction and thus is free to bind another substrate and catalyze a new reaction.
Factors Affecting Enzyme Activity
The active site of the enzyme is sensitive to some environmental factors. The following factors regulate them:
- Temperature: Increasing temperature increases the reaction rate, and lowering a temperature slows down a reaction. However, extremely high temperatures can cause the enzyme to denature and lose its functional activity. There is a specific temperature at which the enzyme activity is at its greatest. The optimum temperature is around 35.5°C for the enzymes in human cells.
- pH: Each enzyme works best within a particular range of pH. Beyond that, it is found to have reduced activity. An extremely high temperature can cause the enzyme to denature
- Enzyme Concentration: Increasing enzyme concentration speeds up a reaction until there are available substrates to bind. Once all the substrates get bound to their enzymes, the reaction shall no longer increase.
- Substrate Concentration: Increasing substrate concentration also increases the reaction rate to a certain point. Once all the enzymes get bound to their substrates, a further increase in the substrate concentration will not affect the reaction rate.
Apart from the above factors, enzyme activity is also affected by ion concentration and the presence or absence of activators and inhibitors. Thus, enzymes work best within a specific range of temperature and pH ranges.
Types and Examples
According to the International Union of Biochemists (IUB), enzymes are classified into six categories or functional classes. They are classified based on the type of reaction they catalyze. They are given below:
| Types | Function | Examples |
|---|---|---|
| 1. Oxidoreductases | Catalyze oxidation-reduction reaction | Pyruvate dehydrogenase, alcohol oxidoreductases, and aldo-keto reductases |
| 2. Transferases | Catalyze transferring of a functional group from one molecule to another | Peptidyl transferase, methyltransferase, and transketolase |
| 3. Hydrolases | Catalyze hydrolysis of proteins, starch, fats, nucleic acids, and other complex biomolecules | Lipases (human pancreatic lipase), peptidases (pepsin), and nucleosidases (kinase) |
| 4. Lyases | Catalyze breaking of a chemical bond by forming double bonds or adding a group to a double bond | Citrate lyase, isocitrate lyase, and hydroxynitrile |
| 5. Isomerases | Catalyze rearrangement causing a structural shift in a molecule and thus changes its shape | Phosphoglucomutase, triose-phosphate isomerase, and phosphate isomerase |
| 6. Ligases | Catalyze the binding or joining of two molecules | Aminoacyl tRNA synthetase, DNA ligase, Succinyl-coenzyme A synthetase |
Functions: Why are Enzymes Important
Enzymes influence most of the biochemical reactions that take place in living organisms, including humans. Enzymes catalyze almost 4,000 such reactions, and the number is expected to be even higher.
Animals, including humans, have a vast number of enzymes working inside the human body. They are widely grouped into metabolic, digestive, and food enzymes.
- Metabolic enzymes help produce energy and detoxify them by breaking down food particles consisting of protein, fat, and carbohydrates.
Examples are carboxylases, dehydrogenases, oxidoreductases, kinases, lyases, transferases, and many more.
- Digestive enzymes catalyze reactions that break down macromolecules – carbohydrates, proteins, and fats into smaller molecules that help the body to produce energy.
Examples are amylase, lipase, maltase, peptidase, and protease.
- Food enzymes are not naturally found in the body of living organisms, but we get their benefit from the food and their supplements.
Examples are cellulase, papain, actinidin, bromelain, and ficin.
Listed below are some more enzymes present in our body with their purpose:
- Lactase: Breaks down lactose, the complex milk sugar
- Pectinase: Breaks down pectin found in fruits and vegetables
- Catalase: Breaks down hydrogen peroxide into water and oxygen
- DNA polymerase: Synthesize DNA from deoxyribonucleotides
- Trypsin: Breaks down protein into amino acids
- Acetylcholinesterase: Breaks down the neurotransmitter acetylcholine
Enzymes are also needed in industry and household products. They are also commercially used to produce fermented products such as beer, wine, and cheese. In the clothing industry, enzymes play a role in reducing impurities in cotton.
FAQs
Ans. Yes, enzymes can be reused.
Ans. Proteolytic enzymes break down protein.
Ans. Amylase breaks down carbohydrates in our bodies.
Ans. DNA helicaseunzips DNA.
Ans. All known enzymes are catalysts, but not all catalysts are enzymes.
Ans. No, enzymes are not used up in a reaction.
Ans. Lipase breaks down fat.
Ans. Amylase breaks down starch.
Ans. No, hemoglobin is not an enzyme. It is a protein.
Ans. The pancreas produces enzymes that break down nutrients.
Ans. Enzymes are primarily organic and are bimolecular, while catalysts can also be inorganic compounds.
Ans. No, enzymes do not have the same shape as their substrates.
Ans. Each enzyme’s active site is specific for one particular substrate – similar to a lock with a specific key. Changing the enzyme’s shape will prevent it from binding to its substrate, thus affecting its function.
Ans. High temperature and extreme pH can denature enzymes.
Ans. Ribosomes are enzymes made in the cell.
Ans. Enzymes only work on their specific substrates because the enzyme’s active site can bind to only a specific substrate – similar to a lock that has a specific key.
Ans. No, enzymes speed up chemical reactions by lowering the activating energy.
Ans. Enzyme inhibitors modify the enzyme’s catalytic properties, thus slowing down the reaction rate and, in some cases, even stopping the reaction.
Ans. The substrate binds to the enzyme at its active site.
Ans. RuBisCO makes up the most abundant protein on earth.
Ans. Yes, enzymes are found in all cells.
Ans. There are approximately 1300 different enzymes found in the human body.
-
References
Article was last reviewed on Thursday, November 11, 2021

Thank you for sharing the wonderful information.