Nitrogen Fixation
Nitrogen is the most abundant element in nature that is essential for plant growth and reproduction. It forms a significant component of chlorophyll, the pigment needed for photosynthesis. It is also found in ATP, nucleic acids, and amino acids, the building blocks of proteins. Although found abundantly, plants can only utilize reduced nitrogen from the soil.
What is Nitrogen Fixation
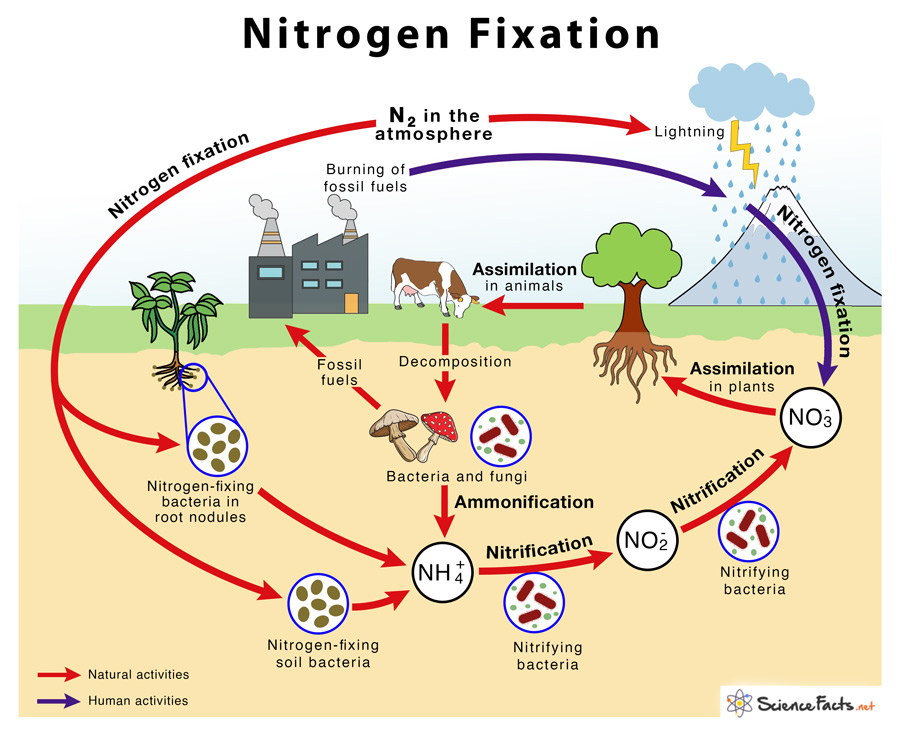
Atmospheric nitrogen fixation or only nitrogen fixation is when atmospheric nitrogen (N2) is converted to ammonia (NH3). It is carried out primarily by soil microorganisms.
Where Does it Occur
Most of the nitrogen fixation occurs naturally in the soil by different nitrogen-fixing bacteria such as Azotobacter, Rhizobium, Azospirillum, and Cyanobacteria. Some amount of nitrogen is also fixed non-biologically by lightning.
Types of Nitrogen Fixation
1) Biological Nitrogen Fixation
Biological nitrogen fixation (BNF) was discovered by Dutch microbiologist Martinus Beijerinck in 1901. It is performed by a group of soil and aquatic bacteria. It is the most common way of nitrogen fixation in nature.
What happens during the process
BNF is a complex process that requires a lot of energy expenditure. Molecular nitrogen consists of two nitrogen atoms joined by a triple covalent bond, making it highly inert and nonreactive. The enzyme nitrogenase present in bacteria breaks this bond to add three hydrogen atoms to each nitrogen atom. Photosynthetic organisms obtain this energy from the oxidation of sugar. In contrast, non-photosynthetic ones derive energy from other organisms on which they rely for food.
The enzyme nitrogenase consists of two soluble proteins: component I and component II. Component I, known as MoFe protein, consists of 2 Mo atoms, 28 to 34 Fe atoms, and 26 to 28 sulfides, together known as an iron-molybdenum cofactor (FeMoco). Component I is composed of two copies of subunits α and β. Component II, known as nitrogenase reductase, is composed of two copies of a single subunit. This protein contains 4 Fe atoms and four acid-labile sulfides (4Fe-4S).
Component I, where substrate binding and reduction occur, binds to ATP and ferredoxin or flavodoxin proteins (Fdx or Fld). The energy for transferring electrons by Fdx or Fld comes from the hydrolysis of ATP. It is a reduction reaction, where electrons are added to N2 to form NH3. Thus, component II’s role is to actively transfer electrons, one at a time, to the component I, coupled to the hydrolysis of ATP.
Chemical Equation
The overall formula for BNF catalyzed by nitrogenase is given below:
N2 + 8H++ 8e− + 16 ATP → 2NH3 + H2 + 16 ADP + 16Pi
Reactants Used and Products Formed
One nitrogen atom binds with three hydrogen atoms to form ammonia (NH3). In this reaction, hydrogen is produced as a byproduct. On average, 21 to 25 ATPs are utilized to fix an atom of nitrogen.
There are three types of biological nitrogen fixation.
A. Symbiotic Nitrogen Fixation
It is the process by which microorganisms fix nitrogen symbiotically through a partnership with a host plant. Here, both plants and the microorganism benefit from each other through the relationship. The plant derives fixed nitrogen from the microorganisms required for their growth, while the microbes get food from the plants.
Examples:
1) Water fern Azolla with the cyanobacterium Anabaena azollae
2) Rhizobium or Bradyrhizobium with leguminous plants such as pea, bean, soybean, and clover
3) Actinorhizal trees and shrubs, such as Alder, with the actinomycete Frankia.
B. Associative Nitrogen Fixation
It is a process where atmospheric nitrogen is converted to ammonia through a casual but close association between a plant and microorganisms.
Examples: Bacterial genera such as Azospirillum, Agrobacterium, Arthrobacter, and Flavobacterium with cereal crops such as rice, wheat, potato, tomato, corn, oats, and barley.
C. Nitrogen Fixation by Free-living Heterotrophs
It is a process where atmospheric nitrogen is fixed by soil-living heterotrophic bacteria that fix nitrogen independently without associating with any other organism. Here, the microorganisms derive energy from the decomposition of organic molecules, performed by other organisms.
Examples: Bacteria of genera Azotobacter, Bacillus, Clostridium, and Klebsiella.
2) Nitrogen Fixation by Lightning
It is a non-biological means of nitrogen fixation. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. In the soil, nitric acid gets converted to nitrate, which is then assimilated by plants.
3) Industrial Nitrogen Fixation
Large-scale nitrogen fixation is done artificially in industries by the Haber process or Haber-Bosch process. German scientist Fritz Haber developed it in the early 1900s. Here, nitrogen (N2) is mixed with hydrogen in the ratio of 1:3 at high pressure of 200 atmospheres and a high temperature of 400 °C. The resulting ammonia is used as synthetic nitrogen in agriculture in the form of nitrogenous fertilizers.
Why is Nitrogen Fixation Necessary
It accomplishes the following purposes in nature:
- Makes nitrogen available to plants and animals for their growth and metabolism
- Helps to continue the nitrogen cycle in nature, where nitrogen fixation is an important step
- Prepares nitrogenous fertilizers and pesticides artificially on a large scale. Thus, helping in crop production and increasing their yield in horticulture.
- Helps to form structural biomolecules such as proteins and nucleic acids (RNA and DNA) that are building blocks of our body
FAQs
Ans. The main difference is that nitrogen fixation reduces nitrogen gas (N2) to ammonia (NH3). In contrast, nitrification is the biological oxidation of ammonia (NH3) to nitrites (NO2–) and nitrates (NO3–).
Ans. Ammonification involves releasing fixed nitrogen as ammonia(NH3) from dead and decaying organic matter by microorganisms. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (N2) is fixed by natural or industrial means to form ammonia (NH3).
-
References
Article was last reviewed on Friday, February 17, 2023