Carnot Engine
Definition: What is the Carnot Engine?
The Carnot engine is an analytical engine that operates on a reversible thermodynamic cycle. It gives the maximum possible efficiency that a heat engine can possess. In a thermodynamic cycle, a system is taken through several stages before returning to its starting initial stage. During this process, the system exchanges heat with the surroundings and perform work. The surroundings consist of two reservoirs, one hot and another cold, that can store a vast amount of heat. They have high heat capacities and are kept at constant temperatures.
The Carnot engine was devised by French engineer and scientist Nicolas Léonard Sadi Carnot in 1824.
Carnot Engine Cycle
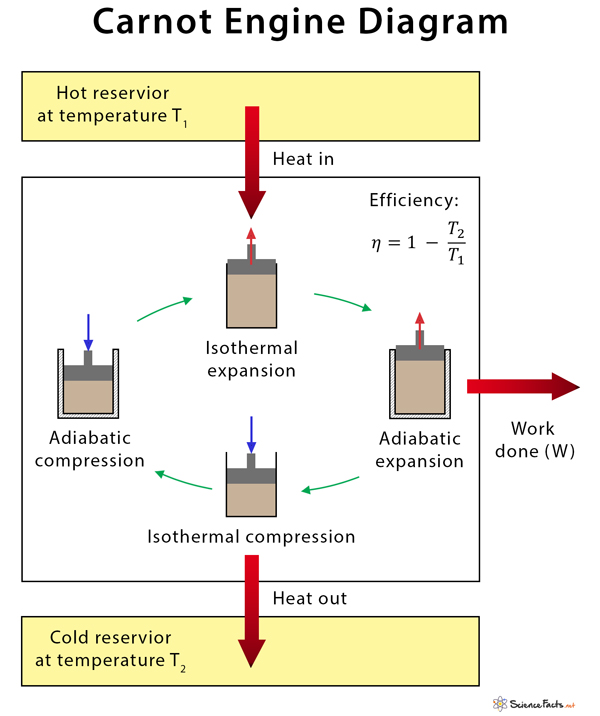
The working of the Carnot engine can be understood by studying its cycle of operations, called the Carnot engine cycle or Carnot cycle. It is a reversible closed thermodynamic cycle in which an ideal gas is kept inside a cylinder with a piston on its top and taken through a series of four successive operations. The sequence of steps in a Carnot cycle is as follows.
- Isothermal expansion: The system takes heat from the hot reservoir, and the gas expands isothermally. During this process, the pressure decreases, and the volume increases while the temperature remains constant at T1.
- Adiabatic expansion: The gas is allowed to expand adiabatically, meaning there is no heat exchange between the system and the surroundings. Here, the temperature decreases to T2, the pressure drops, and the volume increases slightly.
- Isothermal compression: The gas is compressed isothermally. Heat escapes the system into the surroundings and is dumped into the cold reservoir. The temperature remains constant at T2. The pressure increases while the volume decreases.
- Adiabatic compression: Pressure is applied to the gas such that the volume decreases further. The gas is isolated, so there is no heat exchange between the system and the surroundings. This process increases the temperature back to T1.
During the working of the Carnot engine, the system is taken from temperature T1 to T2 and then back from temperature T2 to T1. It is best represented by a pressure-volume (PV) diagram.
Carnot Refrigerator
The thermodynamic cycle that is observed in the Carnot engine is a reversible process. The engine can be acted upon by an external force to transfer heat. Thus, the Carnot engine works like a refrigerator. Heat is sucked out of the cold reservoir inside a fridge. Electrical energy (work input) is supplied from the wall outlet. There is some waste heat dumped into the kitchen (surrounding).
Efficiency of Carnot Engine
All heat engines perform work and are defined by their efficiencies. In physics, efficiency is the ratio of work done (W) to the amount of heat the system takes (Qh).
Qc is the heat that is wasted and dumped into the cold reservoir. The following formula gives the efficiency of a Carnot engine.
The conceptual thinking of the Carnot cycle is that it establishes the maximum possible efficiency for any heat engine cycle operating between T1 and T2. The reason is that there is no friction between the piston and the walls of the cylinder. It runs on reversible processes, which yields maximum work output for a given heat input. However, the Carnot engine cannot achieve 100% thermal efficiency because some waste heat is always produced during its operation.
Principle of Carnot Engine: Carnot Theorem
The principle of the Carnot engine, also called the Carnot theorem, states that “Any system working between two given temperatures, T1 (hot reservoir) and T2 (cold reservoir), can never have an efficiency more than the Carnot engine working between the same reservoirs respectively.”
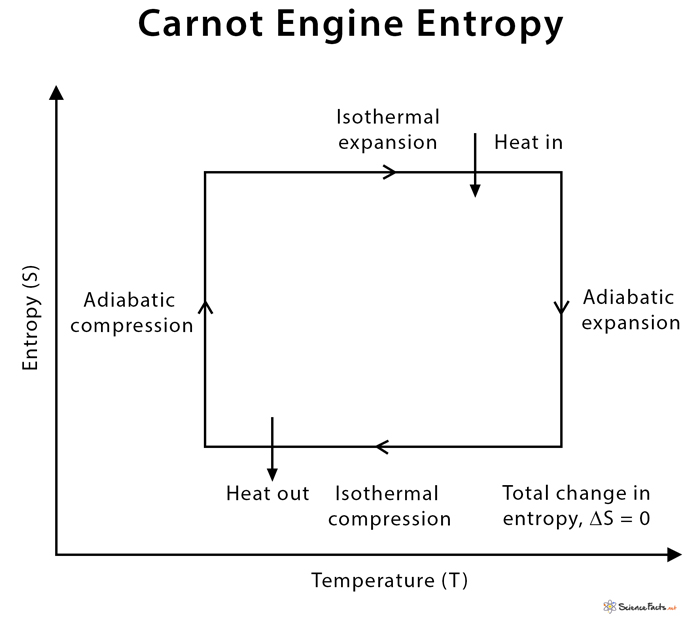
Carnot Engine Entropy
Another useful quantity that measures the disorder of the system is entropy. It is the measure of a system’s thermal energy per unit temperature that is unavailable for useful work. Since the Carnot cycle is reversible, its entropy change is zero.
Applications of Carnot Engine
Carnot engine has practical applications in many thermal devices and machines, mostly because they require a temperature gradient to do mechanical work. Here are some of the applications, including in real-world modeling.
- To evaluate real engines in the context of their theoretical maximum efficiency, especially during the design.
- As a theoretical reference cycle to compare the efficiencies of the actual Otto (petrol) and Diesel cycles.
- To convert oceanic thermal energy into work.
However, the Carnot engine is impractical because the heat transfer into the engine in the isothermal process is too slow to be of practical value.
Carnot Engine Animation
-
References
Article was last reviewed on Saturday, September 30, 2023